The Absorbance Coefficient Calculator is a vital tool used to determine the absorbance of a solution. This is essential in various fields such as chemistry, biology, and environmental science. By inputting the molar absorptivity, concentration of the solution, and path length of the sample, this calculator provides accurate absorbance values. This helps in analyzing the properties of solutions and understanding their behavior under different conditions.
Formula of Absorbance Coefficient Calculator
The formula for calculating the absorbance coefficient is:
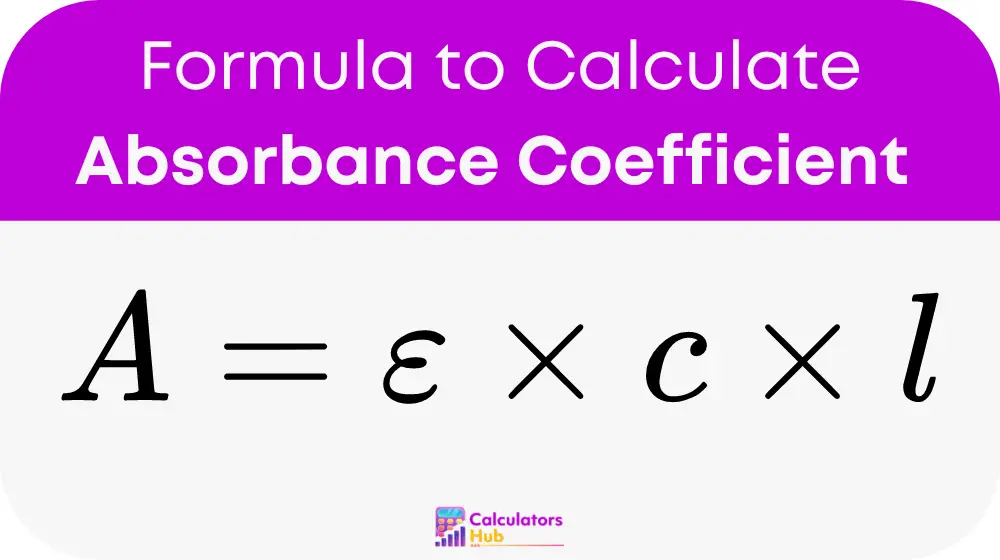
where:
- A is the absorbance
- ε (epsilon) is the molar absorptivity or absorbance coefficient
- c is the concentration of the solution
- l is the path length of the sample
This formula is fundamental in spectroscopy and helps in quantifying how much light is absorbed by a solution at a given wavelength.
Conversion Table
To assist users, here is a table of common values and their corresponding absorbance coefficients. This can help users quickly reference values without performing manual calculations.
| Concentration (M) | Path Length (cm) | Absorbance Coefficient (ε) | Absorbance (A) |
|---|---|---|---|
| 0.1 | 1 | 100 | 10 |
| 0.2 | 1 | 100 | 20 |
| 0.1 | 2 | 100 | 20 |
| 0.5 | 1 | 100 | 50 |
| 0.1 | 1 | 200 | 20 |
Example of Absorbance Coefficient Calculator
Let’s consider an example to illustrate how to use the Absorbance Coefficient Calculator.
Example Calculation:
Given:
- ε (molar absorptivity) = 150 M^-1 cm^-1
- c (concentration) = 0.05 M
- l (path length) = 1 cm
Using the formula:
A = ε * c * l A = 150 * 0.05 * 1 A = 7.5
Thus, the absorbance of the solution is 7.5.
Most Common FAQs
Molar absorptivity, also known as the absorbance coefficient, is a measure of how strongly a chemical species absorbs light at a given wavelength. It is expressed in units of M^-1 cm^-1.
To determine the concentration, rearrange the absorbance formula: c = A / (ε * l). By measuring the absorbance and knowing the molar absorptivity and path length, you can calculate the concentration.
The path length, typically measured in centimeters, is the distance that light travels through the solution. It directly affects the absorbance value, as a longer path length results in higher absorbance for the same concentration.