The Force of Attraction Between Two Ions Calculator is an invaluable tool designed to simplify the process of understanding ionic interactions. At its core, this calculator uses a fundamental principle of physics to measure the force exerted between two charged ions. Whether for educational purposes, research, or practical applications in the fields of chemistry and physics, this calculator provides clear, accurate, and immediate insights into the forces at play at the atomic level.
Formula
The calculation at the heart of this tool is based on Coulomb’s Law, which is represented as:
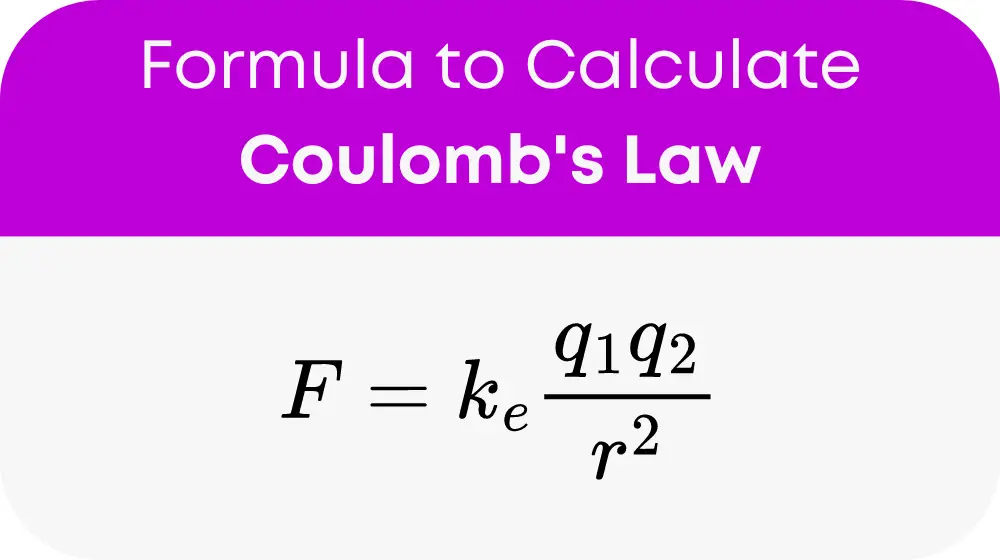
where:
Fis the force of attraction (in Newtons),kis Coulomb’s constant (approximately8.99 x 10^9 Nm^2/C^2),q1andq2are the charges of the two ions (in Coulombs),dis the distance between the centers of the ions (in meters).
General Terms and Conversions Table
To assist users further, a table of common terms and necessary conversions is provided. This table is designed to help users quickly reference key information without needing to calculate each time, making the calculator even more user-friendly and accessible.
| Term | Description | Value |
|---|---|---|
Coulomb’s Constant (k) | The fundamental constant in electrostatics | 8.99 x 10^9 Nm^2/C^2 |
| Electron Charge | Charge of a single electron | -1.602 x 10^-19 C |
| Proton Charge | Charge of a single proton | +1.602 x 10^-19 C |
Ion Charge (q) | Typical ion charges | -1e, -2e, +1e, +2e (e=electron charge) |
Distance (d) | Common distances in atomic structures | Varied; often in the range of 10^-10 meters |
This table serves as a quick reference for the most commonly encountered values and terms in calculations related to the force of attraction between ions.
Example of Force of Attraction Between Two Ions Calculator
Consider two ions, one with a charge of +1e (proton charge) and another with a charge of -1e (electron charge), separated by a distance of 1 x 10^-10 meters. By applying the formula:
F = (8.99 x 10^9) * (1.602 x 10^-19) * (-1.602 x 10^-19) / (1 x 10^-10)^2
we can calculate the force of attraction between these ions. This example helps illustrate the direct application of the calculator in practical scenarios.
Most Common FAQs
A1: Yes, the calculator is versatile and can be use for ions with any charge magnitude. As long as the values are entered in Coulombs.
A2: The calculator is highly accurate, utilizing Coulomb’s Law directly. However, the precision of the results depends on the accuracy of the input values.
A3: While there’s no strict limit, the calculator is design for typical distances observe in atomic and molecular structures, generally within the range of a few nanometers or less.