The Effective Charge Calculator is a powerful tool used in atomic physics and chemistry to estimate the effective nuclear charge (Z_eff) experienced by an electron in a multi-electron atom. The value of Z_eff helps describe how tightly an electron is held by the nucleus after accounting for the shielding effect caused by other electrons.
This calculator falls under the Chemistry and Atomic Physics Calculators category.
Z_eff plays a key role in understanding atomic structure, periodic trends (such as ionization energy and atomic radius), and chemical behavior. It helps students, researchers, and educators model how electrons interact within atoms and predict reactivity trends more accurately.
formula of Effective Charge Calculator
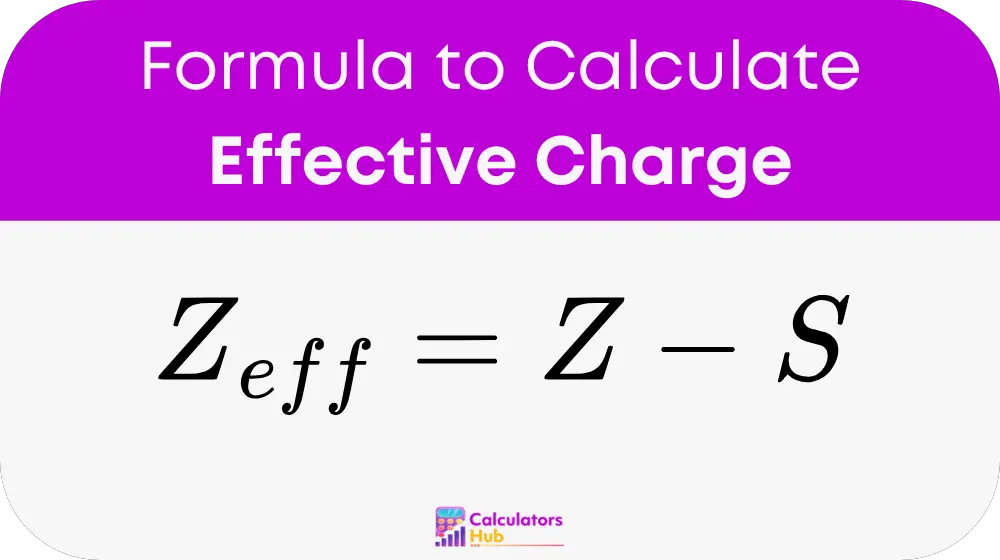
Variable Definitions:
Z_eff:
Effective Nuclear Charge (measured in elementary charge units), which is the net positive charge felt by an electron after accounting for electron shielding.
Z:
Atomic Number, which equals the number of protons in the nucleus of the atom.
S:
Shielding Constant, which is the sum of the shielding contributions from all other electrons, determined using Slater’s Rules.
Slater’s Rules for Calculating S (Shielding Constant):
Slater's Rules provide a step-by-step method to estimate the shielding effect.
- Group electrons into sets:
- (1s), (2s, 2p), (3s, 3p), (3d), (4s, 4p), etc.
- Apply these shielding values:
- For each electron in the same group as the target electron:
→ Add 0.35 (use 0.30 if in the 1s group) - For each electron in the (n−1) level (one shell lower):
→ Add 0.85 - For each electron in levels (n−2) or lower:
→ Add 1.00 - For d- and f-block electrons:
- All electrons in the same group contribute 0.35
- All inner electrons contribute 1.00
- For each electron in the same group as the target electron:
Sum all the contributions to get S, and subtract from Z to get Z_eff.
Reference Table for Effective Nuclear Charges
| Element | Atomic Number (Z) | Shielding Constant (S, approx.) | Effective Nuclear Charge (Z_eff) |
|---|---|---|---|
| Hydrogen (H) | 1 | 0.30 | 0.70 |
| Helium (He) | 2 | 0.30 | 1.70 |
| Lithium (Li, 2s electron) | 3 | 1.30 | 1.70 |
| Beryllium (Be, 2s electron) | 4 | 1.95 | 2.05 |
| Fluorine (F, 2p electron) | 9 | 4.85 | 4.15 |
| Neon (Ne, 2p electron) | 10 | 5.10 | 4.90 |
This table gives quick values for commonly studied elements, helping users save time when analyzing trends.
Example of Effective Charge Calculator
Let’s calculate the effective nuclear charge for a 2p electron in a fluorine (F) atom.
Step 1: Determine Atomic Number (Z):
Fluorine has Z = 9
Step 2: Determine Shielding Constant (S):
According to Slater’s Rules:
- Electrons in the same group (2s, 2p): 4 electrons × 0.35 = 1.40
- Electrons in (n−1) shell (1s): 2 electrons × 0.85 = 1.70
- Total S = 1.40 + 1.70 = 3.10
Step 3: Apply Formula:
Z_eff = 9 − 3.10 = 5.90
Result:
The effective nuclear charge on a 2p electron in fluorine is approximately 5.90
Most Common FAQs
A: Z_eff indicates how strongly an electron is attracted to the nucleus after considering the repulsion from other electrons. A higher Z_eff means a stronger pull on the electron, affecting size, ionization energy, and reactivity.
A: Slater’s Rules provide good approximations for educational and conceptual use. For highly accurate results, quantum chemistry methods are used, but Slater’s method is widely accepted for general use.
A: Yes, but extra care is needed when dealing with d- and f-orbitals. The rules for shielding contributions change slightly, and it's important to group electrons correctly.