A Critical Temperature Calculator is a tool used to determine the critical temperature of a substance, which is the highest temperature at which a gas can be liquefied by pressure alone. Above this temperature, the substance cannot exist as a liquid, regardless of the pressure applied. This value is crucial in thermodynamics, material science, and engineering applications involving phase transitions, refrigeration, and high-pressure gas systems.
Formula of Critical Temperature Calculator
To calculate the Critical Temperature (T_c), the following formula is used:
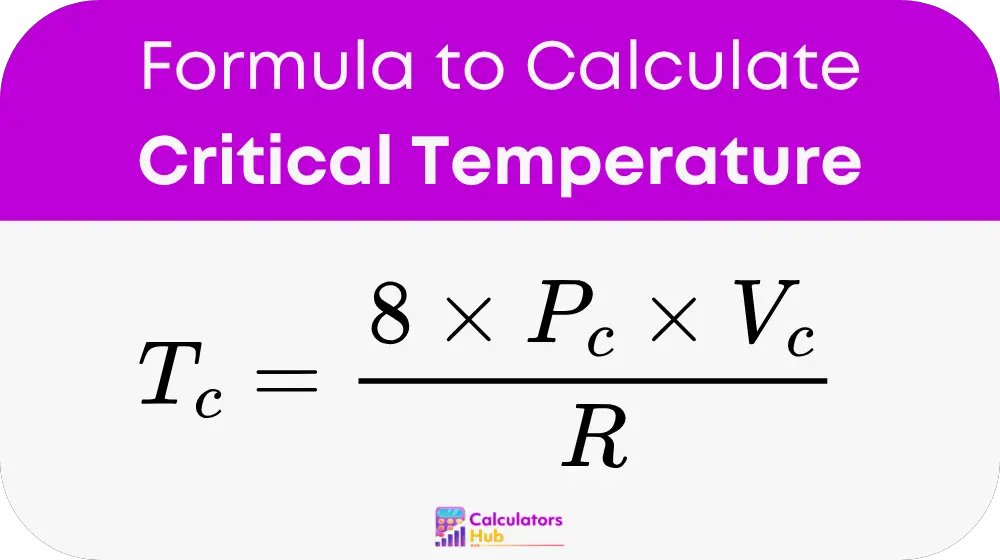
Where:
- T_c = Critical Temperature (in Kelvin, K)
- P_c = Critical Pressure (in Pascals, Pa or atm)
- V_c = Critical Volume (in cubic meters, m³ or liters, L)
- R = Universal Gas Constant (8.314 J/mol·K or 0.0821 L·atm/mol·K)
This equation is derived from the van der Waals equation and is widely applied in the study of gases and their behavior under critical conditions.
Importance of Critical Temperature
Understanding critical temperature is essential in various fields, including:
- Chemical Engineering: Designing and optimizing industrial processes.
- Refrigeration Systems: Managing phase transitions of refrigerants.
- Supercritical Fluids: Used in CO₂ extraction and high-pressure applications.
- Cryogenics: Studying the behavior of gases at extremely low temperatures.
General Reference Table for Common Substances
| Substance | Critical Temperature (K) | Critical Pressure (atm) | Critical Volume (L/mol) |
|---|---|---|---|
| Oxygen (O₂) | 154.6 K | 50.4 atm | 0.073 L/mol |
| Nitrogen (N₂) | 126.2 K | 33.9 atm | 0.089 L/mol |
| Carbon Dioxide (CO₂) | 304.2 K | 73.8 atm | 0.094 L/mol |
| Water (H₂O) | 647.1 K | 218.3 atm | 0.056 L/mol |
| Methane (CH₄) | 190.6 K | 45.9 atm | 0.099 L/mol |
Example of Critical Temperature Calculator
Let's calculate the critical temperature of carbon dioxide (CO₂) using known values:
- P_c = 73.8 atm
- V_c = 0.094 L/mol
- R = 0.0821 L·atm/mol·K
Using the formula:
T_c = (8 * 73.8 * 0.094) / 0.0821
T_c = (55.3536) / 0.0821 ≈ 304.3 K
Thus, the critical temperature of CO₂ is approximately 304.3 K, which aligns with the known reference values.
Most Common FAQs
Critical temperature helps determine the conditions under which a gas can be liquefied. It is essential in designing industrial processes involving phase transitions and gas handling.
Supercritical fluids exist beyond their critical temperature and pressure, where they exhibit unique properties such as low viscosity and high diffusivity, making them useful in extraction, decaffeination, and chemical processing.
Above the critical temperature, a substance cannot be liquefied by pressure alone. Instead, it forms a supercritical fluid with properties of both gas and liquid, leading to applications in power generation, pharmaceuticals, and materials processing.