The Number Of Atoms In Each Sample Calculator is an invaluable tool for students, educators, and professionals in the sciences. It simplifies the process of calculating the total number of atoms in any chemical sample. By inputting the number of moles present in a sample, the calculator uses Avogadro's number to determine the total atoms, bridging a critical gap between theoretical chemistry and practical application.
Formula of Number Of Atoms In Each Sample Calculator
The calculator operates on a straightforward principle encapsulated by the formula:
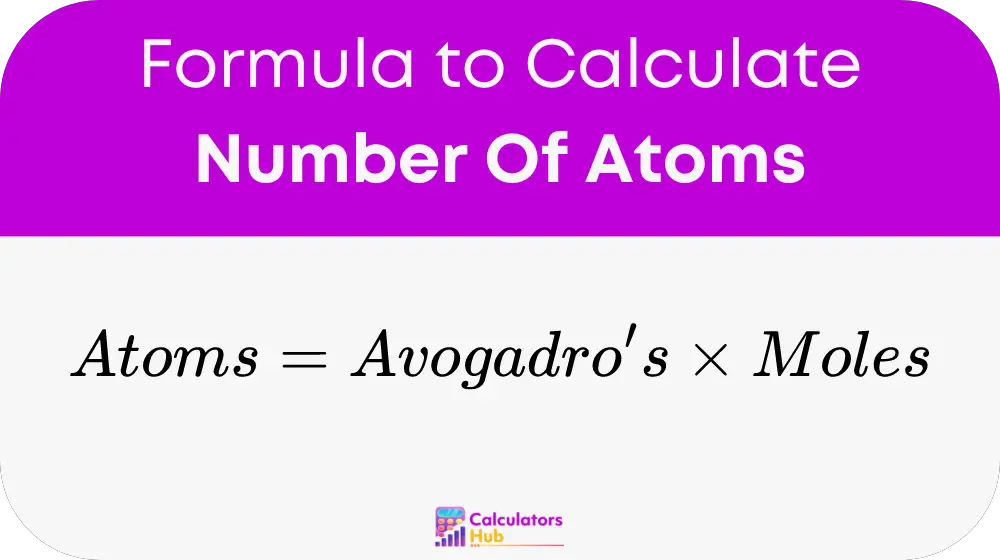
Where:
- Number of atoms is the total number of atoms in the sample.
- Avogadro's number is a constant, approximately 6.022 × 10^23 atoms/mole, which is the number of units in one mole of any substance.
- Number of moles is the measure of substance in moles.
Understanding and applying this formula allows for precise scientific calculations and enhances comprehension of material quantities on a molecular level.
Useful Conversion Table
To further aid our users, here is a conversion table that provides quick reference points:
| Substance | Molar Mass | Example Moles | Atoms Calculated |
|---|---|---|---|
| Water (H2O) | 18 g/mol | 1 mole | 6.022 x 10^23 atoms |
| Carbon (C) | 12 g/mol | 1 mole | 6.022 x 10^23 atoms |
| Sodium Chloride (NaCl) | 58.44 g/mol | 1 mole | 6.022 x 10^23 atoms |
This table helps bypass calculations for common substances frequently encountered in laboratories.
Example of Number Of Atoms In Each Sample Calculator
Let's calculate the number of atoms in 2 moles of carbon dioxide (CO2):
- Determine moles of CO2: 2 moles.
- Use the formula:
Number of atoms = 6.022 x 10^23 atoms/mole × 2 moles = 12.044 x 10^23 atoms
This example demonstrates how to use the calculator to estimate the number of atoms in common chemical compounds.
Most Common FAQs
Avogadro's number is the number of constituent particles (usually atoms or molecules) in one mole of a given substance, set at approximately 6.022 x 10^23.
The number of moles can be determined by dividing the mass of the substance by its molar mass.
Yes, this calculator is universal as long as the number of moles and the substance's molar mass are known.