The KC Calculator is designed to convert the equilibrium constant expressed in terms of partial pressure (KP) to its equivalent in concentration (KC). This conversion is vital for calculations involving chemical equilibria in solutions where concentrations are used instead of gas pressures.
Formula of KC Calculator from KP
To calculate the equilibrium constant KC from KP, use the following formula:
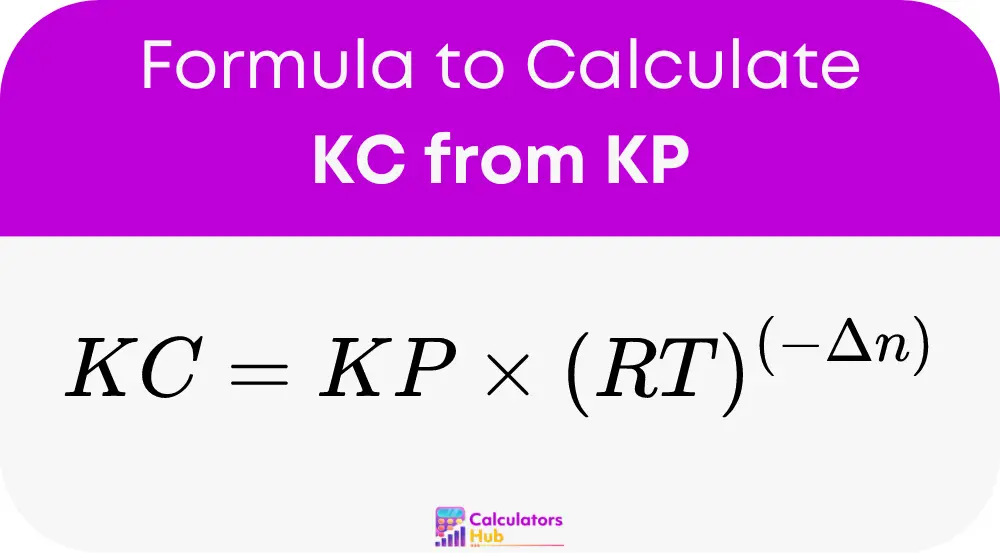
Where:
- KC is the equilibrium constant in terms of concentration (molarity).
- KP is the equilibrium constant in terms of partial pressure.
- R is the ideal gas constant (0.0821 L·atm·mol⁻¹·K⁻¹).
- T is the temperature in Kelvin.
- Δn is the change in the number of moles of gas (moles of gaseous products minus moles of gaseous reactants).
Step-by-Step Guide to Calculate KC
Step 1: Determine KP
Obtain the equilibrium constant value in terms of partial pressure from experimental data or literature.
Step 2: Convert Temperature to Kelvin
If the temperature is provided in Celsius, convert it to Kelvin by adding 273.15.
Step 3: Calculate Δn
Determine the net change in moles of gas by considering the stoichiometry of the reaction.
Step 4: Use the Formula
Plug the values of KP, R, T, and Δn into the formula to compute KC.
Conversion Table and Tools
Here’s a basic table showing typical values for KC calculations under various conditions for the same reaction:
| Condition | KP (atm−2−2) | Temp (°C) | Temp (K) | Δ𝑛Δn | KC (M−2−2) |
|---|---|---|---|---|---|
| Standard Conditions | 120 | 25 | 298 | -2 | 50720 |
| High Temperature | 150 | 500 | 773 | -2 | 168300 |
| Low Temperature | 100 | -20 | 253 | -2 | 38440 |
This table provides quick reference values for estimating KC under different thermal conditions without detailed calculations.
Example of KC Calculator from KP
Consider the chemical reaction where nitrogen gas (N2) reacts with hydrogen gas (H2) to form ammonia (NH3):
N2(g) + 3H2(g) -> 2NH3(g)
For this reaction at a temperature of 500 K, the equilibrium constant in terms of partial pressure (KP) is known to be 150 atm^-2. We need to convert this to the equilibrium constant in terms of concentration (KC).
Steps to Calculate KC:
- KP Value: 150 atm^-2
- Temperature (T): 500 K
- Change in Moles of Gas (Delta n):
- Moles of reactants: 1 N2 + 3 H2 = 4 moles
- Moles of products: 2 NH3 = 2 moles
- Delta n = 2 – 4 = -2
- Calculation:
- Formula used: KC = KP * (RT)^(-Delta n)
- R (ideal gas constant) = 0.0821 L·atm·mol^-1·K^-1
- Calculation of (RT)^(-Delta n): (0.0821 * 500)^-2
- Substituting values and calculating KC:
- KC = 150 * (0.0821 * 500)^2
- KC = 150 * 20.525^2
- KC = 150 * 421.450625
- KC = 63217.59375 M^-2
This example shows how to convert KP to KC for this reaction, considering the given temperature and change in moles.
Most Common FAQs
A1: Changes in temperature affect the value of T in the formula, thus directly impacting KC.
A2: Yes, when Δn is zero, KP equals KC since the exponent term becomes 1.
A3: Δn determines the exponent in the formula, influencing the magnitude and direction of the effect on KC.