The Equilibrium Potential for Na+ Calculator computes the voltage across the cellular membrane at which sodium ions are in electrochemical equilibrium. This means that the electrical force counterbalances the chemical gradient of Na+, preventing net movement across the membrane. This calculation is essential for anyone studying cellular electrical activity and is particularly relevant in neuroscience and pharmacology.
Formula of Equilibrium Potential for Na+ Calculator
To calculate the equilibrium potential for sodium (E_Na), the following formula is used:
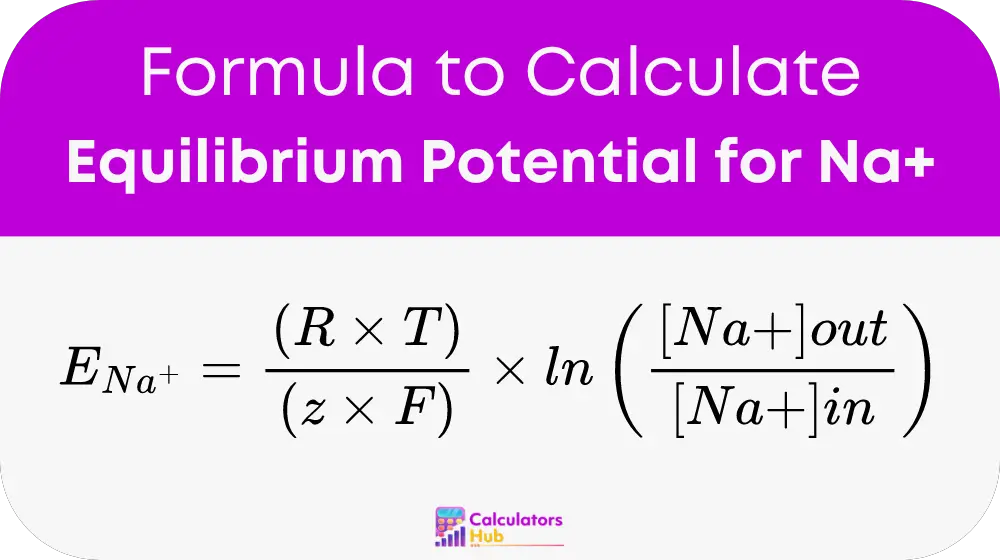
Here,
ENarepresents the equilibrium potential for Na+ in millivolts (mV)Ris the universal gas constant (8.314 J/K mol)Tis the absolute temperature in Kelvinzis the valence of the ion (+1 for Na+)Fis Faraday’s constant (96485 C/mol)[Na+]outis the concentration of sodium ions outside the cell[Na+]inis the concentration inside the cell.
This formula incorporates the Nernst equation, highlighting the dependency of equilibrium potential on ion concentration and temperature.
Table of Typical Ion Concentrations
For practical use, here is a table displaying typical concentrations of sodium ions inside and outside various cells:
| Cell Type | [Na+]in (mM) | [Na+]out (mM) |
|---|---|---|
| Neurons | 10 | 145 |
| Muscle Cells | 12 | 140 |
| Epithelial Cells | 15 | 145 |
This table aids users in performing quick calculations or estimations without needing to gather extensive data.
Example of Equilibrium Potential for Na+ Calculator
Let’s calculate the equilibrium potential for a typical neuron at body temperature (37°C):
- Convert temperature to Kelvin: T = 37 + 273.15 = 310.15 K
- Use the ion concentrations from the table: [Na+]in = 10 mM, [Na+]out = 145 mM
- Substitute into the formula:
- E_Na = (8.314 * 310.15) / (1 * 96485) * ln(145 / 10)
- = 61.54 mV
This example demonstrates the calculator’s utility in predicting neuron behavior under physiological conditions.
Most Common FAQs
The concentration of sodium ions inside and outside the cell and the temperature are the primary factors.
Higher temperatures increase the kinetic energy of ions, potentially altering the equilibrium potential.
The equilibrium potential for an ion cannot be zero unless the concentrations of the ion inside and outside the cell are equal, which is physiologically improbable.