The Absorption Frequency Calculator helps you determine the frequency at which a molecule absorbs energy. This frequency is crucial in various scientific fields, such as spectroscopy, chemistry, and physics. By calculating the absorption frequency, you can understand the energy levels within a molecule and how it interacts with electromagnetic radiation.
Formula of Absorption Frequency Calculator
The formula to calculate the absorption frequency (ν) is:
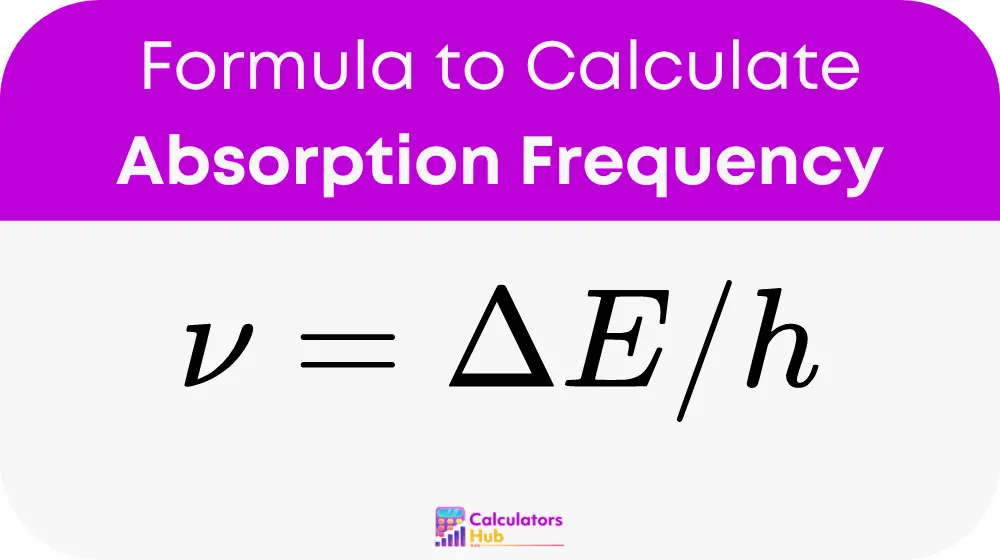
Where:
- ν is the absorption frequency in Hertz (Hz)
- ΔE is the energy difference between the two energy levels in Joules (J)
- h is Planck's constant, approximately 6.626 x 10^-34 Joule seconds (Js)
To use this formula, divide the energy difference (ΔE) by Planck's constant (h) to get the absorption frequency (ν).
Common Terms and Conversion Table
Here is a table with general terms and common conversions related to absorption frequency:
| Term | Definition |
|---|---|
| Absorption Frequency (ν) | The frequency at which a molecule absorbs energy, measured in Hertz (Hz). |
| Energy Difference (ΔE) | The difference in energy between two levels, measured in Joules (J). |
| Planck's Constant (h) | A physical constant that relates the energy of a photon to its frequency, approximately 6.626 x 10^-34 Joule seconds (Js). |
Example of Absorption Frequency Calculator
Let's go through an example to illustrate how to use the Absorption Frequency Calculator.
Suppose we have an energy difference (ΔE) of 3.0 x 10^-19 Joules between two energy levels. To find the absorption frequency (ν), we use the formula:
ν = ΔE / h
Substituting the given values:
ν = (3.0 x 10^-19 J) / (6.626 x 10^-34 Js)
ν ≈ 4.52 x 10^14 Hz
So, the absorption frequency is approximately 4.52 x 10^14 Hz.
Most Common FAQs
A1: The absorption frequency is important because it helps identify the energy levels within a molecule. This information is crucial for understanding molecular structures and interactions in various scientific fields.
A2: Planck's constant (h) is used to relate the energy difference (ΔE) between two levels to the absorption frequency (ν). It is a fundamental constant in quantum mechanics.
A3: Yes, the calculator can be used for any molecule as long as the energy difference between the two levels is known. It is a versatile tool for various applications in spectroscopy and molecular analysis.