The Accelerated Stability Calculator is a tool used to estimate the shelf life of a product under accelerated conditions. This calculator is important in various industries, such as pharmaceuticals, food, and cosmetics, where understanding the stability of a product is crucial for ensuring its safety and effectiveness. By using accelerated conditions, such as higher temperatures, the calculator helps predict how a product will behave over time, allowing companies to make informed decisions about product formulation and storage.
Formula of Accelerated Stability Calculator
The accelerated stability calculator uses the Arrhenius equation to determine the rate of a chemical reaction, which in turn helps predict the product's stability. The formula is as follows:
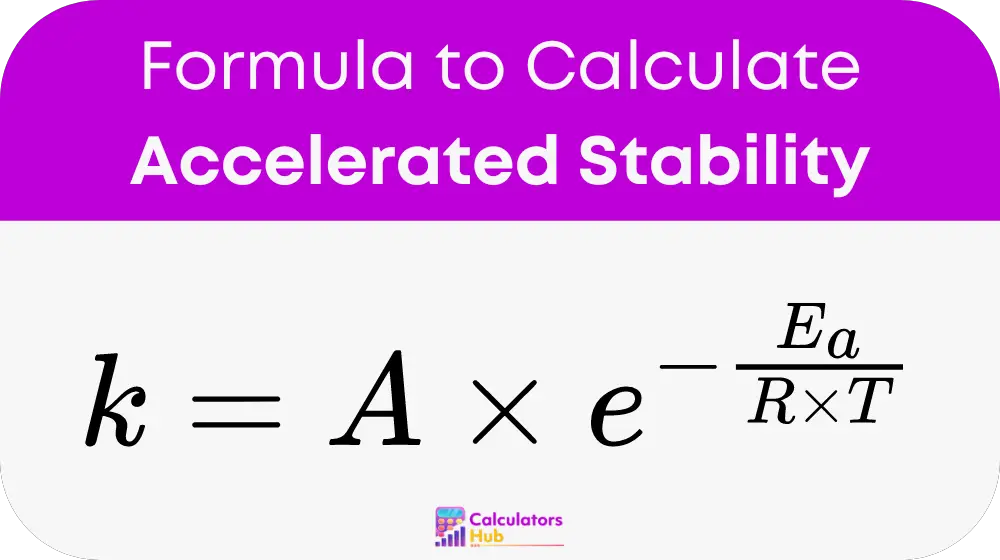
Where:
- k = rate constant
- A = frequency factor (pre-exponential factor)
- Ea = activation energy (in joules per mole)
- R = universal gas constant (8.314 J/mol·K)
- T = temperature (in Kelvin)
This equation shows that the rate of a chemical reaction increases with temperature, which is why accelerated conditions can provide insights into the long-term stability of a product in a shorter amount of time.
General Terms and Conversions Table
| Term | Definition | Conversion |
|---|---|---|
| Rate Constant (k) | A measure of the speed of a chemical reaction | N/A |
| Frequency Factor (A) | A constant that indicates the frequency of collisions between reactant molecules | N/A |
| Activation Energy (Ea) | The minimum energy required for a chemical reaction to occur | 1 kJ = 1000 J |
| Universal Gas Constant (R) | A physical constant that appears in the Arrhenius equation | 8.314 J/mol·K |
| Temperature (T) | The measure of thermal energy | °C = K - 273.15 |
Example of Accelerated Stability Calculator
Let's consider an example to illustrate the use of the Accelerated Stability Calculator.
Suppose we have a product with the following parameters:
- Activation Energy (Ea): 50,000 J/mol
- Frequency Factor (A): 1 x 10^13 s^-1
- Storage temperature: 298 K (25°C)
- Accelerated temperature: 318 K (45°C)
Step-by-Step Calculation:
- Calculate the rate constant at the storage temperature: k_298 = 1 x 10^13 * e^(-50,000 / (8.314 * 298))
- Calculate the rate constant at the accelerated temperature: k_318 = 1 x 10^13 * e^(-50,000 / (8.314 * 318))
- Compare the rate constants to determine the acceleration factor.
By following these steps, you can estimate how the product's stability changes under accelerated conditions.
Most Common FAQs
The accuracy of the Accelerated Stability Calculator depends on the quality of the input data and the assumptions made during the calculations. While it provides a good estimate, real-time stability testing is recommended for confirmation.
The calculator is widely applicable but may not be suitable for products that undergo complex degradation pathways or have components that interact in unpredictable ways. It's always best to consult with a stability expert.
Accelerated conditions can provide quick insights but may not perfectly mimic real-world storage conditions. Factors like humidity, light exposure, and physical handling can also affect product stability and should be considered.