The Effective Nuclear Charge Calculator helps determine the net positive charge felt by an electron in a multi-electron atom. This net charge is known as the effective nuclear charge (Z_eff) and plays a key role in understanding atomic behavior, electron configurations, ionization energies, and periodic trends like atomic radius and electronegativity.
This calculator falls under the Chemistry Calculators category and is widely used in academic chemistry, molecular physics, and materials science. By calculating Z_eff, students and researchers can better understand how strongly the nucleus attracts electrons and how shielding by inner electrons affects this attraction.
formula of Effective Nuclear Charge Calculator
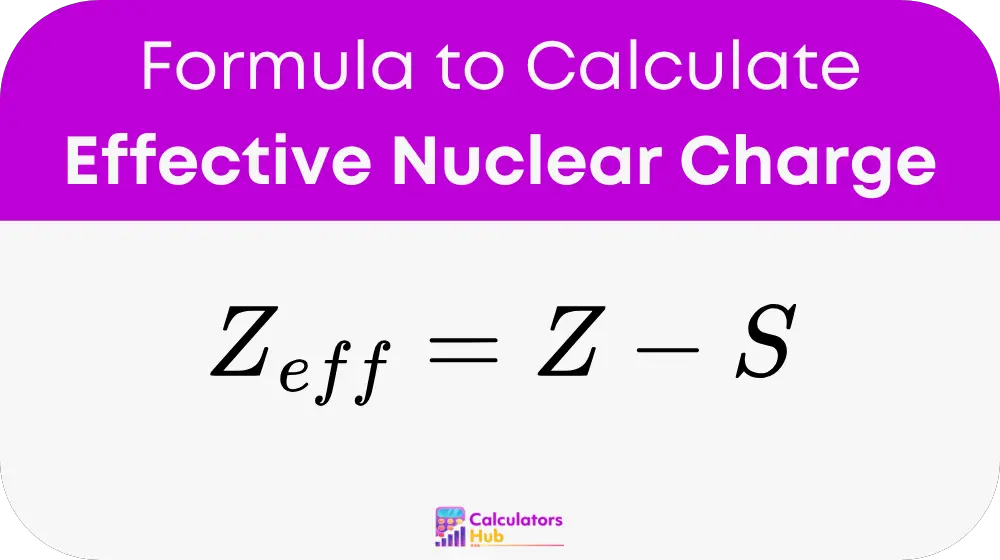
Variables:
-
Z_eff:
Effective Nuclear Charge — the net charge acting on an electron, measured in units of elementary charge (e). -
Z:
Atomic Number — the number of protons in the nucleus of the atom. -
S:
Shielding Constant — the average number of inner electrons that reduce the effect of nuclear charge on the electron being considered. It is usually calculated using Slater’s Rules.
Notes:
- Slater’s Rules provide a systematic way to estimate S by accounting for electron repulsions from other electrons in the atom.
- A higher Z_eff means stronger attraction between the nucleus and electrons, which typically results in smaller atomic size and higher ionization energy.
Reference Table: Common Effective Nuclear Charges by Element Type
Below is a simplified reference table showing approximate effective nuclear charges for some representative elements. This helps users quickly understand general trends without calculating every time.
| Element | Atomic Number (Z) | Approx. Shielding (S) | Z_eff = Z – S |
|---|---|---|---|
| Hydrogen (H) | 1 | 0.0 | 1.0 |
| Lithium (Li) | 3 | 1.7 | 1.3 |
| Carbon (C) | 6 | 2.5 | 3.5 |
| Oxygen (O) | 8 | 3.5 | 4.5 |
| Neon (Ne) | 10 | 4.5 | 5.5 |
| Sodium (Na) | 11 | 10.2 | 0.8 |
| Chlorine (Cl) | 17 | 10.0 | 7.0 |
Tip: Elements in the same group tend to have similar Z_eff for their valence electrons, even as atomic number increases.
Example of Effective Nuclear Charge Calculator
Scenario:
Find the effective nuclear charge for a valence electron in a phosphorus (P) atom.
Step 1: Identify Atomic Number
Z = 15
Step 2: Estimate Shielding Constant using Slater’s Rules
S ≈ 10.2
Step 3: Use the formula
Z_eff = Z – S
Z_eff = 15 – 10.2 = 4.8
Result:
The effective nuclear charge for a valence electron in phosphorus is approximately 4.8 elementary charges. This value explains why phosphorus holds its valence electrons more tightly than elements earlier in the period.
Most Common FAQs
A: It explains trends in the periodic table like atomic size, ionization energy, and electronegativity. It shows how tightly the nucleus holds its outer electrons.
A: No. Since shielding only reduces the nuclear pull, the effective nuclear charge will always be less than or equal to Z.
< /div>A: Use Slater’s Rules, which group electrons into shells and sublevels and assign specific shielding values for each group.