A Moonshine Dilution Calculator is designed to provide precise assistance in diluting moonshine to your desired alcohol concentration. This is crucial not only for achieving your preferred flavor and strength but also for ensuring the drink is safe to consume.
Formula of Moonshine Dilution Calculator
Let’s dive into the formula used in moonshine dilution:
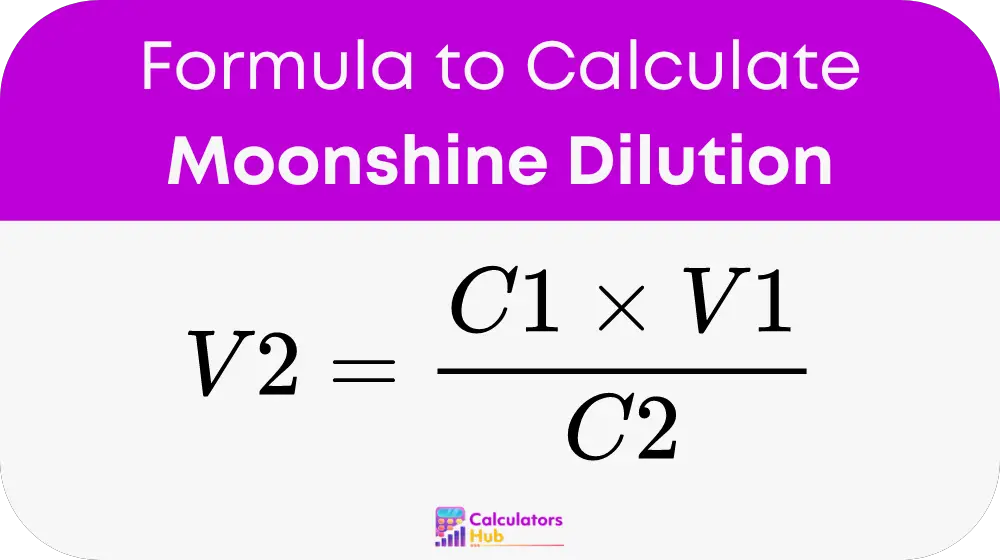
Where:
- C1 is the initial concentration of your moonshine in percent alcohol by volume (% ABV).
- V1 represents the initial volume of your moonshine, which can be in liters, gallons, or another unit.
- C2 is the final concentration you desire for your moonshine in % ABV.
- V2 is the final volume of moonshine you will achieve after dilution, in the same unit as V1.
Understanding each part of this formula is key to diluting your moonshine correctly.
Moonshine Dilution Conversion Table
| Initial ABV (%) | Desired ABV (%) | Initial Volume | Additional Water Required |
|---|---|---|---|
| 80 | 40 | 1 gallon | 1 gallon |
| 80 | 40 | 5 gallons | 5 gallons |
| 80 | 40 | 10 gallons | 10 gallons |
| 60 | 30 | 1 gallon | 1 gallon |
| 60 | 30 | 5 gallons | 5 gallons |
| 60 | 30 | 10 gallons | 10 gallons |
| 50 | 25 | 1 gallon | 1 gallon |
| 50 | 25 | 5 gallons | 5 gallons |
| 50 | 25 | 10 gallons | 10 gallons |
Example of Moonshine Dilution Calculator
Let’s walk through a practical example: Suppose you have 5 gallons of moonshine at 80% ABV and want to reduce it to 40% ABV. Using our formula:
V2 = (80% * 5 gallons) / 40% = 10 gallons
You will need to add enough water to reach a total volume of 10 gallons, effectively diluting your moonshine to the desired 40% ABV.
Most Common FAQs
Always ensure that the units of V1 and V2 match, and convert your measurements accordingly before applying the formula.
Always dilute in a well-ventilated area, wear protective gear, and ensure accurate measurement for safe handling.
While designed for moonshine, this calculator can be used for any spirit where dilution to a specific ABV is needed. However, always consider the specific characteristics of the spirit you are diluting.