The Fenske Equation Calculator helps chemical engineers determine the minimum number of theoretical stages needed for binary or multicomponent distillation under total reflux. This tool is critical for designing or analyzing distillation columns, particularly during the conceptual or feasibility phase. By using composition data of distillate and bottoms and relative volatility, users can quickly estimate separation requirements. It assumes no loss of material and constant volatility throughout the column, making it ideal for quick process evaluations and column sizing.
="wp-block-heading">formula of Fenske Equation Calculator
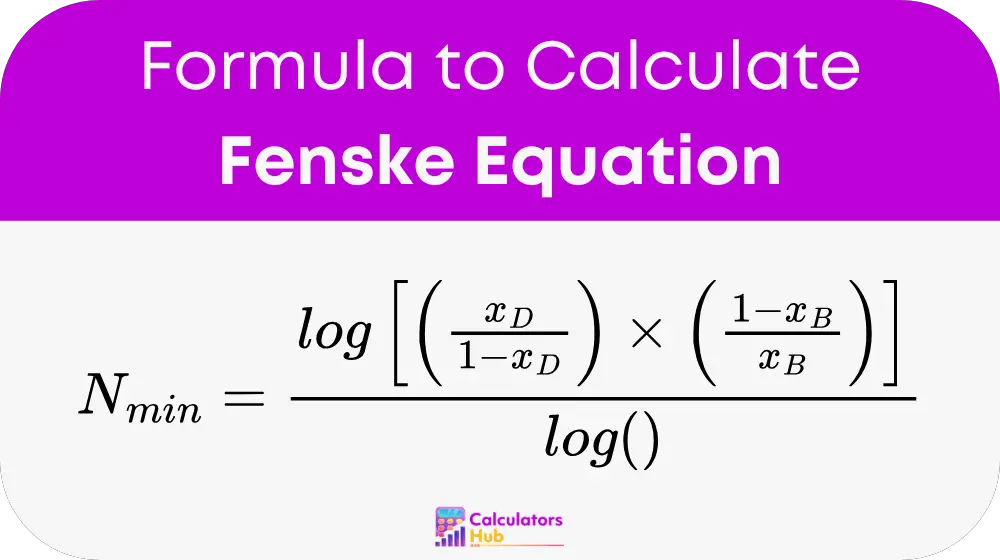
Where:
Nₘᵢₙ = minimum number of theoretical stages
x_D = mole fraction of the more volatile component in the distillate
x_B = mole fraction of the more volatile component in the bottoms
ᾱ = average relative volatility of the more volatile component relative to the less volatile one
log = logarithm (can be base 10 or natural, but must be consistent)
Supporting Notes:
- Only applies under total reflux conditions (no product removal)
- Assumes constant relative volatility throughout the column
- For multicomponent mixtures, the equation is used for the light key vs heavy key components
- High ᾱ means easier separation (fewer stages needed)
="wp-block-heading">Table of Typical Values and Volatility Ratios
| Component Pair | Relative Volatility (ᾱ) | Common Use Case |
|---|---|---|
| Benzene / Toluene | 2.5 | Petrochemical separations |
| Methanol / Water | 1.1 | Azeotropic mixture (harder split) |
| Ethanol / Water | 1.8 | Bioethanol recovery |
| Acetone / Methanol | 2.0 | Solvent recovery |
| Hexane / Heptane | 1.4 | Hydrocarbon purification |
These values help engineers understand how volatile differences affect stage requirements.
Example of Fenske Equation Calculator
Scenario:
A distillation process aims to separate benzene (more volatile) from toluene.
Given:
x_D = 0.95 (benzene in distillate)
x_B = 0.05 (benzene in bottoms)
ᾱ = 2.5 (relative volatility of benzene to toluene)
Step 1:
Numerator = log[(0.95 / 0.05) × (0.95 / 0.05)] = log(361) ≈ 2.558
Step 2
:Denominator = log(2.5) ≈ 0.398
Step 3:
Nₘᵢₙ = 2.558 / 0.398 ≈ 6.43 stages
This means at least 7 theoretical stages are required under total reflux to achieve the desired separation.
="wp-block-heading">Most Common FAQs
A: It means no product is withdrawn during operation. All condensed vapor is returned as reflux, maximizing separation efficiency and simplifying calculations.
A: It gives the theoretical minimum stages. Actual columns require more stages to account for inefficiencies, partial reflux, and non-ideal behavior.
A: The Fenske equation still applies but only between the light key and heavy key components that define the separation boundary.