The Solution Freezing Point Calculator is designed to help users determine how the presence of a solute affects the freezing temperature of a solvent. This calculation is essential for anyone working in fields where the purity of substances or the behavior of solutions under different temperatures is relevant.
Formula of Solution Freezing Point Calculator
The core of our calculator is based on the fundamental formula:
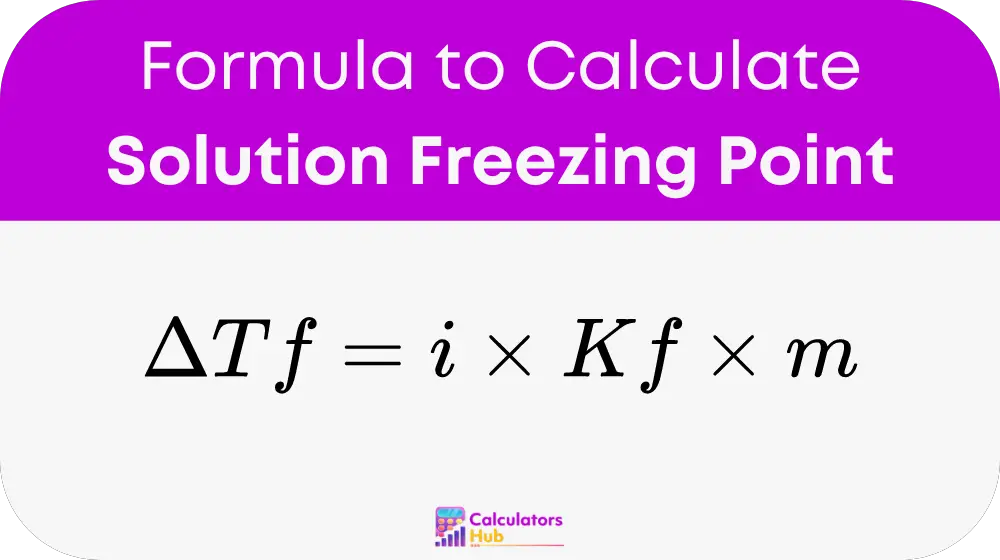
Where:
- ΔTf is the decrease in the freezing point (in degrees Celsius),
- i represents the van’t Hoff factor, indicating the number of particles the solute splits into in the solution,
- Kf is the cryoscopic constant, which varies with the solvent,
- m stands for molality, measured as moles of solute per kilogram of solvent.
Understanding each part of this formula is key to utilizing the calculator effectively.
Table of Freezing Points for Various Aqueous Solutions
| Solute | Molality (mol/kg) | van’t Hoff Factor (i) | Freezing Point Depression (ΔTf) | Freezing Point (°C) |
|---|---|---|---|---|
| Sodium Chloride (NaCl) | 0.5 | 2 | 1.86 | -1.86 |
| Sodium Chloride (NaCl) | 1.0 | 2 | 3.72 | -3.72 |
| Calcium Chloride (CaCl2) | 0.5 | 3 | 2.79 | -2.79 |
| Calcium Chloride (CaCl2) | 1.0 | 3 | 5.58 | -5.58 |
| Sucrose (C12H22O11) | 0.5 | 1 | 0.93 | -0.93 |
| Sucrose (C12H22O11) | 1.0 | 1 | 1.86 | -1.86 |
| Ethanol (C2H5OH) | 0.5 | 1 | 0.93 | -0.93 |
| Ethanol (C2H5OH) | 1.0 | 1 | 1.86 | -1.86 |
| Potassium Chloride (KCl) | 0.5 | 2 | 1.86 | -1.86 |
| Potassium Chloride (KCl) | 1.0 | 2 | 3.72 | -3.72 |
Example of Solution Freezing Point Calculator
Let’s walk through a practical example: Suppose we need to determine the freezing point depression of an aqueous NaCl solution where NaCl dissociates into two particles, and the solution’s molality is 0.5 mol/kg. Using our formula:
ΔTf = 2 * 1.86 * 0.5 = 1.86°C
This result shows the freezing point of water will be depressed by 1.86°C when NaCl is dissolved at the given concentration.
Most Common FAQs
A: It’s the lowering of a solvent’s freezing point due to the addition of a solute.
A: The impact depends on the solute’s ability to dissociate in the solvent, as represented by the van’t Hoff factor.
A: Always ensure the purity of your solvent and solute, and carefully measure the molality of the solution.