Relative Atomic Mass Calculator helps in determining the relative atomic mass (also known as atomic weight) of an element.This value is vital for various calculations in chemistry, including reactant and product quantification in chemical reactions.
Formula of Relative Atomic Mass Calculator
The formula for calculating relative atomic mass is:
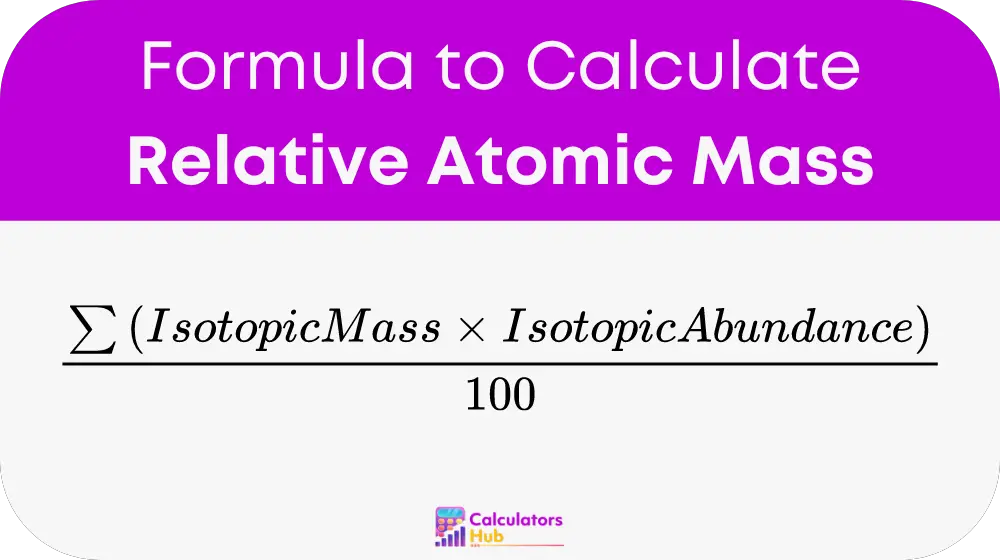
Here’s a breakdown of the formula:
- Σ (sigma): Represents the summation of all isotopes of the element.
- Isotope mass: The mass of a specific isotope, expressed in atomic mass units (amu).
- Isotope abundance: The natural abundance percentage of an isotope, converted into a decimal for calculation.
- / 100: Converts the percentage abundance to a decimal.
Table of Common Calculations
Below is a table featuring common elements and their relative atomic masses calculated for quick reference:
| Element | Relative Atomic Mass (amu) |
|---|---|
| Hydrogen | 1.008 |
| Carbon | 12.011 |
| Oxygen | 15.999 |
This table serves as a quick reference to avoid manual calculations every time.
Example of Relative Atomic Mass Calculator
To make concepts more clear let’s look at an example. Let’s calculate the relative atomic mass of Chlorine, which has two main isotopes: Chlorine-35 and Chlorine-37.
- Isotope masses and abundances:
- Chlorine-35: 34.969 amu, 75.78% abundance
- Chlorine-37: 36.966 amu, 24.22% abundance
- Calculation:
- (34.969 x 75.78 + 36.966 x 24.22) / 100 = 35.453 amu
This example illustrates how to use the calculator to derive meaningful results effectively.
Most Common FAQs
The calculator is highly accurate, provided the isotopic mass and abundance values are up-to-date and correctly input.
Yes, the calculator works for any element, as long as you have the necessary isotopic data.
The same formula applies, just include all isotopes in the summation.