The Molarity Calculator Tocris serves as a crucial tool for chemists and researchers who deal with chemical solutions daily. It allows users to quickly compute the mass of a solute needed to achieve a specific molar concentration in a given volume of solvent. This functionality is indispensable in laboratories where accuracy and efficiency are paramount.
Formula of Molarity Calculator Tocris
At the heart of the calculator is a straightforward formula:
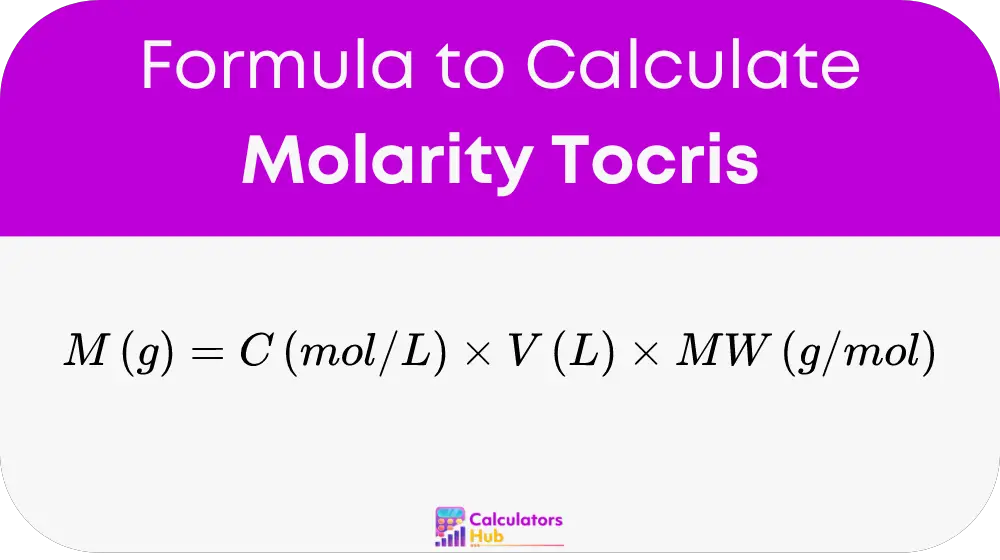
Let’s break this down:
- Mass (g): This is the weight of the solute that needs to be dissolve.
- Concentration (mol/L): The desired concentration of the solution, indicating how many moles of solute per liter of solution are required.
- Volume (L): The total volume of the solution in which the solute will be dissolved.
- Molecular Weight (g/mol): This represents the mass of one mole of the solute.
Useful Conversion Table
To aid in your calculations, here’s a handy table with essential conversions:
| Conversion | Details |
|---|---|
| Grams to Moles | Divide by the molecular weight |
| Liters to Milliliters | Multiply by 1,000 |
| Moles to Particles | Multiply by Avogadro’s number |
Example of Molarity Calculator Tocris
Imagine you need to prepare 2 liters of a 0.5 M NaCl solution. Here’s how you use the calculator:
- Determine the molecular weight of NaCl (58.44 g/mol).
- Apply the formula: 0.5 mol/L x 2 L x 58.44 g/mol = 58.44 grams.
- Measure 58.44 grams of NaCl and dissolve in 2 liters of water.
Most Common FAQs
A: Use a precision scale for solid components and volumetric flasks for liquid measurements.
A: Recalculate the mass of the solute using the adjusted volume in the formula to maintain the desired concentration.
A: Yes, it can be adjusted to accommodate various solvents, though users should verify the solvent compatibility with the solute.