Molar absorbance, a key concept in spectroscopy, measures how well a chemical species absorbs light at a given wavelength. This calculator simplifies the complex calculations involved in determining molar absorptivity, a parameter essential for quantifying the concentration of solutions accurately.
Formula of Molar Absorbance Calculator
The cornerstone of understanding molar absorbance lies in the Beer-Lambert Law:
A = ε * c * l
Where:
- A is the absorbance (unitless),
- ε is the molar absorptivity (L/mol·cm),
- c is the concentration of the solution (mol/L),
- l is the path length of the cuvette (cm).
To find the molar absorptivity, rearrange the formula:
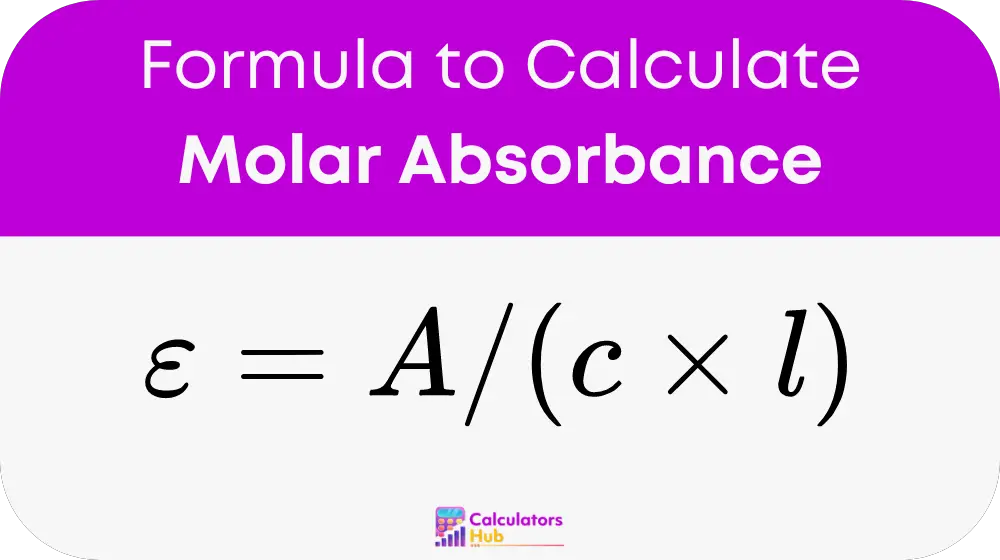
This rearrangement allows researchers to calculate the molar absorptivity if the other variables are known, facilitating a deeper understanding of the solution's properties.
General Terms Table
To aid in your scientific endeavors, below is a table of terms frequently encountered when dealing with molar absorbance:
| Term | Definition |
|---|---|
| Absorbance (A) | The measure of light absorption. |
| Molar Absorptivity (ε) | Molar effectiveness of a substance in absorbing light. |
| Concentration (c) | Amount of solute per unit volume of solution. |
| Path Length (l) | Distance light travels through the solution. |
Example of Molar Absorbance Calculator
Consider a solution with an absorbance of 0.5, a concentration of 0.1 mol/L, and a path length of 1 cm. Using our formula:
ε = 0.5 / (0.1 * 1) = 5 L/mol·cm
This example illustrates the straightforward application of the Molar Absorbance Calculator to real-world scenarios.
Most Common FAQs
Molar absorptivity can vary widely, typically ranging from 0 to 100,000 L/mol·cm, depending on the substance and conditions.
The path length directly influences the absorbance; longer paths result in higher absorbance, assuming other variables remain constant.
It is best used for clear solutions as turbidity can scatter light and affect absorbance readings.