The Keq calculator is designed to provide a quick and accurate calculation of the equilibrium constant from the concentrations of reactants and products involved in a chemical reaction. This tool is essential for chemists who need to predict the outcome of reactions and for educational purposes where understanding chemical equilibrium dynamics is necessary.
Formula of Keq Calculator
The equilibrium constant (Keq) is calculated using the formula:
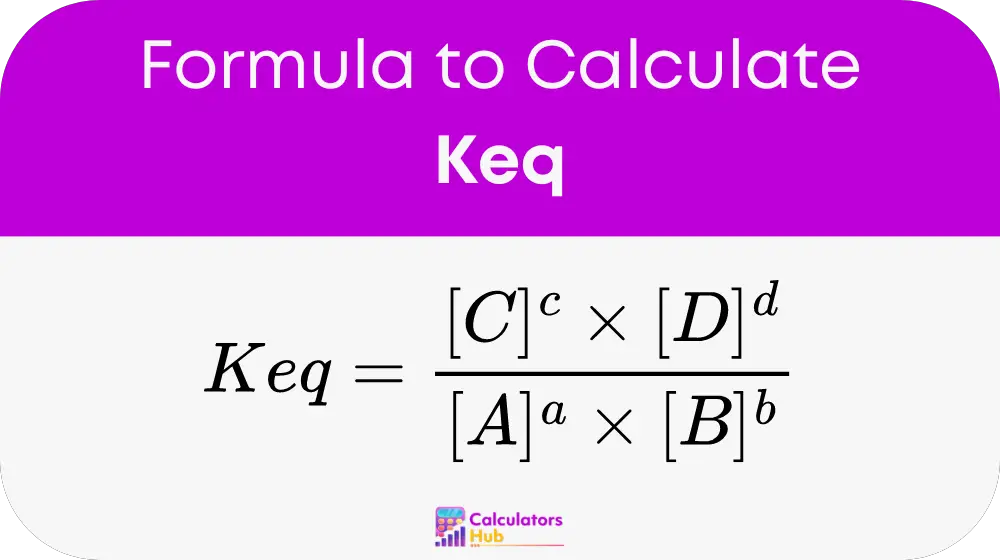
Where:
- A, B, C, D are the chemical species involved in the reaction.
- a, b, c, d are the stoichiometric coefficients of these species.
This formula reflects how the concentrations of chemical species at equilibrium influence the reaction’s direction and extent.
Table of General Terms and Conversion Factors
To aid in the practical application of the Keq calculator, the following table lists common terms and conversion factors:
| Term | Definition | Example |
|---|---|---|
| Concentration | Amount of a substance per defined space | mol/L (Molarity) |
| Stoichiometric Coefficient | Number indicating the ratio in a balanced equation | as seen in the formula |
| Reaction Quotient | Ratio of product concentrations to reactant concentrations before reaching equilibrium | Used in predictive calculations |
This table serves as a quick reference to enhance understanding and application of the Keq formula without needing detailed calculations for each scenario.
Example of Keq Calculator
Consider a hypothetical reaction where hydrogen gas (H2) and iodine gas (I2) react to form hydrogen iodide (HI):
H2 + I2 -> 2HI
Using hypothetical concentrations at equilibrium:
- [HI] = 0.2 M
- [H2] = 0.1 M
- [I2] = 0.1 M
The Keq would be calculate as:
Keq = ([HI]^2) / ([H2] * [I2]) = (0.2^2) / (0.1 * 0.1) = 4
This example illustrates how the Keq calculator simplifies determining the equilibrium constant, providing insights into the reaction dynamics.
Most Common FAQs
A1: Keq provides a quantitative measure of a reaction’s tendency to proceed to completion or reverse, essential for predicting and controlling chemical processes.
A2: Yes, Keq is temperature-dependent. Changes in temperature can shift the equilibrium position, affecting the Keq value.