The GFM Calculator is an essential tool for chemists, pharmacists, educators, and students, facilitating accurate calculations of the gram formula mass of a compound—a critical step in many chemical analyses. By inputting the elements of a compound and their respective quantities, the calculator outputs the total gram formula mass, which is the sum of the atomic masses of all atoms in a molecular formula.
Formula of GFM Calculator
To utilize the GFM Calculator effectively, one must understand the underlying formula it uses:
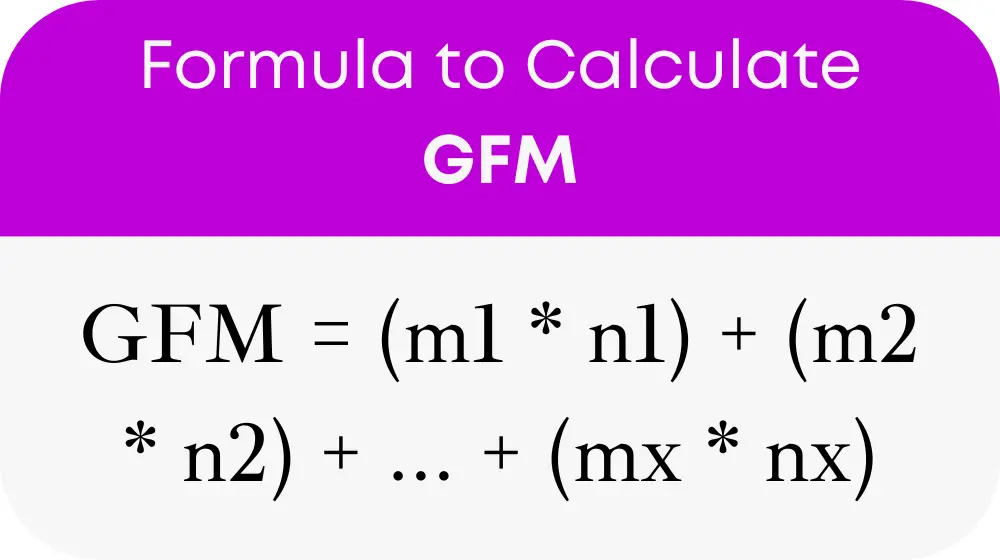
Where GFM is the gram formula mass, m1, m2, ..., mx are the atomic masses of the elements, and n1, n2, ..., nx are the number of atoms of each element in the compound. This formula ensures that all aspects of the compound's molecular structure are accounted for in the mass calculation.
Table of Common Calculations
For convenience, below is a table of common compounds and their GFM values calculated using the GFM Calculator:
| Compound | Formula | GFM (g/mol) |
|---|---|---|
| Water | H₂O | 18.015 |
| Carbon Dioxide | CO₂ | 44.01 |
| Sodium Chloride | NaCl | 58.44 |
| Glucose | C₆H₁₂O₆ | 180.156 |
This table acts as a quick reference to save time and ensure accuracy when these common compounds are involved in experiments or calculations.
Example of GFM Calculator
Let's calculate the GFM of water (H₂O):
- Identify the elements: Hydrogen (H) and Oxygen (O).
- Count the atoms: 2 Hydrogen atoms and 1 Oxygen atom.
- Find atomic masses: Hydrogen = 1.008 g/mol, Oxygen = 16.00 g/mol.
- Apply the formula: GFM=(2×1.008)+(1×16.00)=18.016 g/mol
The GFM of water is approximately 18.016 g/mol, which is essential for various calculations in chemistry.
Most Common FAQs
Gram Formula Mass (GFM) is the total mass of all the atoms in a compound's molecular formula, expressed in grams per mole. It is crucial for stoichiometric calculations, which are fundamental in preparing solutions and performing chemical reactions accurately.
For complex compounds, break down the compound into identifiable elements and their respective counts, input these into the calculator, and use the formula provided to calculate the GFM accurately.
Common mistakes include miscounting the atoms of each element, using incorrect atomic masses, and inputting elements incorrectly. Always double-check your inputs and ensure all elements are accounted for.