The Electrolysis Voltage Calculator is a scientific tool design to estimate the minimum voltage required to drive an electrochemical reaction, commonly known as electrolysis. Electrolysis is the process by which electrical energy is use to induce a non-spontaneous chemical reaction, such as splitting water into hydrogen and oxygen.
This calculator helps chemists, engineers, educators, and researchers determine the potential difference needed to overcome the thermodynamic and practical barriers in an electrolysis setup. It accounts for standard electrode potentials, temperature, electron transfer, and concentration of ions, allowing users to optimize their electrochemical experiments or industrial processes.
Formula of Electrolysis Voltage Calculator
To calculate the voltage required for electrolysis, the Nernst equation is use in a slightly modified form to accommodate the full electrolysis cell:
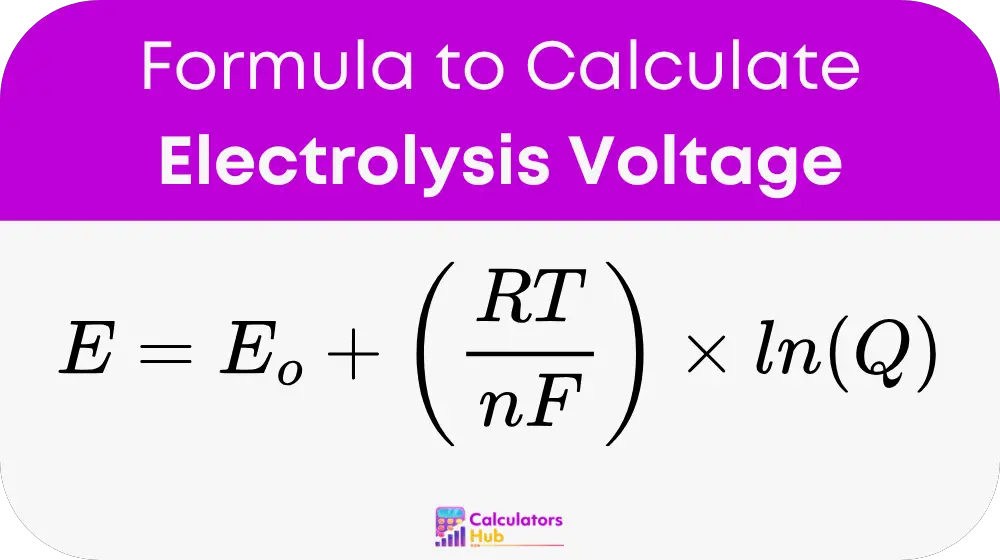
Where:
- E is the electrolysis cell potential (in volts, V)
- E₀ is the standard electrode potential (in volts, V)
- R is the universal gas constant (8.314 J/mol·K)
- T is the absolute temperature (in Kelvin, K)
- n is the number of electrons transferred in the electrochemical reaction
- F is the Faraday constant (96,485 C/mol)
- Q is the reaction quotient, representing the ratio of product and reactant concentrations
This equation allows for adjusting the voltage based on temperature and chemical conditions, providing a more accurate estimate than standard potential alone.
Understanding the Components
- E₀ values are known for standard redox reactions and available in electrochemical series tables.
- Q is calculated from the concentrations or partial pressures of reactants and products.
- T in Kelvin must be converted from Celsius using T(K) = T(°C) + 273.15.
- n depends on the specific redox process. For example, in water electrolysis, n = 2 for H₂ generation.
General Terms Related to Electrolysis Voltage Calculation
The following table provides essential terms and definitions frequently associated with electrolysis calculations. Understanding these helps users apply the calculator effectively.
| Term | Definition |
|---|---|
| Electrolysis | A process that uses electricity to drive a chemical change or reaction |
| Electrode Potential (E₀) | The voltage of a half-cell under standard conditions (1 M, 25°C, 1 atm) |
| Cell Potential (E) | The actual voltage required for the full electrolysis reaction |
| Reaction Quotient (Q) | The ratio of concentrations of products to reactants at any given moment |
| Faraday Constant (F) | The amount of electric charge per mole of electrons (96,485 C/mol) |
| Temperature (T) | Must be used in Kelvin in all electrochemical equations |
| Electrons Transferred (n) | The number of electrons moved per molecule of reactant in redox reaction |
| Overpotential | Additional voltage required due to inefficiencies and real-world losses |
These terms form the foundation for understanding how voltage in electrolysis is calculated and how practical systems behave.
Example of Electrolysis Voltage Calculator
Let’s walk through a practical example of calculating electrolysis voltage for the decomposition of water into hydrogen and oxygen.
Given:
- Standard electrode potential for the overall reaction (E₀) = 1.23 V
- Temperature (T) = 298 K (25°C)
- Number of electrons transferred (n) = 2
- Concentrations are standard, so Q = 1
Step 1: Use the Formula
E = 1.23 + [(8.314 × 298) / (2 × 96485)] × ln(1)
E = 1.23 + (2477.572 / 192970) × 0 = 1.23 + 0
Final Answer: E = 1.23 V
In this case, the electrolysis voltage remains at the standard potential because the reaction quotient is 1 and there are no deviations from standard conditions. However, in real setups, overpotential and inefficiencies usually increase the required voltage to around 1.8–2.0 V.
Most Common FAQs
The required voltage for electrolysis depends on the standard electrode potential, the temperature of the system, the concentration of ions involved (via the reaction quotient), and the number of electrons transferred. In practical settings, overpotential also plays a significant role.
Real systems have inefficiencies such as resistance in the electrolyte, electrode surface limitations, and side reactions. These are accounted for as overpotentials, requiring a higher actual voltage than predicted by the theoretical formula.
Yes, it can be lowered by optimizing the electrode material, increasing temperature, improving electrolyte conductivity, and maintaining ideal concentration levels. Using catalysts at the electrodes also helps reduce overpotential.