The Concentration Coefficient Calculator helps determine the concentration of a substance in a solution using absorbance data. This calculation is based on the Beer-Lambert law, a fundamental principle in chemistry and spectroscopy. It is widely used in fields like biochemistry, pharmacology, and environmental science to analyze the composition of samples and solutions.
Formula of Concentration Coefficient Calculator
The calculation follows the Beer-Lambert law:
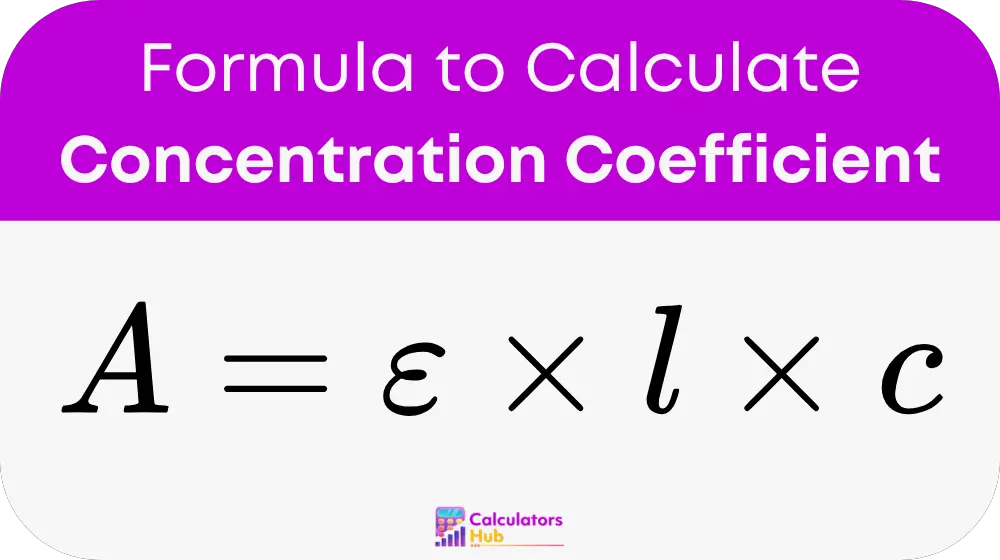
Where:
- A: Absorbance, measured using a spectrophotometer.
- ε: Molar absorptivity (a constant specific to the substance, typically in L/(mol·cm)).
- l: Path length of the light through the solution (cm, usually 1 cm for standard cuvettes).
- c: Concentration of the substance (mol/L).
To calculate concentration (c), the formula is rearranged as:
c = A / (ε × l)
Steps to Calculate
- Measure Absorbance (A) using a spectrophotometer.
- Determine Molar Absorptivity (ε) for the substance, which can be obtained from reference materials or experimental data.
- Measure or identify the Path Length (l), which is often the width of the cuvette used in spectrophotometry.
- Substitute the known values into the rearranged formula to solve for concentration.
Pre-calculated Table for Common Scenarios
Below is a table for quick reference using typical values for ε, l, and A:
| Absorbance (A) | Molar Absorptivity (ε, L/(mol·cm)) | Path Length (l, cm) | Concentration (c, mol/L) |
|---|---|---|---|
| 0.5 | 1000 | 1 | 0.0005 |
| 1.0 | 2000 | 1 | 0.0005 |
| 0.3 | 1500 | 1 | 0.0002 |
| 0.8 | 1000 | 1 | 0.0008 |
Example of Concentration Coefficient Calculator
Scenario
A sample solution exhibits an absorbance (A) of 0.6 at a specific wavelength. The molar absorptivity (ε) of the substance at this wavelength is 1500 L/(mol·cm), and the path length (l) is 1 cm. Calculate the concentration of the solution.
Step-by-Step Calculation
- List Known Values:
- A = 0.6
- ε = 1500 L/(mol·cm)
- l = 1 cm
- Apply the Formula:
c = A / (ε × l)
c = 0.6 / (1500 × 1)
c = 0.0004 mol/L
The concentration of the solution is 0.0004 mol/L.
Most Common FAQs
It simplifies the process of determining the concentration of a solution using absorbance data and the Beer-Lambert law, saving time in laboratory analyses.
Yes, but you must know the molar absorptivity (ε) for the specific substance at the wavelength used for measurement.
Ensure accurate measurements of absorbance, use the correct value for molar absorptivity, and verify the path length of the cuvette to prevent calculation errors.