The Common Ion Effect Calculator is a tool designed to simplify the process of determining the equilibrium concentration of ions in a solution when a common ion is introduced. This phenomenon occurs when an ionic compound is dissolved in a solution that already contains one of its ions, leading to a reduction in solubility due to Le Chatelier’s Principle. The calculator uses mathematical formulas to analyze how the presence of a common ion impacts the equilibrium concentration of the dissolved species. It is widely used in chemistry to study solubility, buffer solutions, and equilibrium processes.
This tool is particularly helpful for students, chemists, and researchers when they are working on solubility equilibria or need to calculate the effects of ion interactions in solutions efficiently.
Formula of Common Ion Effect Calculator
The calculator employs the quadratic formula to determine the equilibrium concentration (C) of the ion of interest. The formula is:
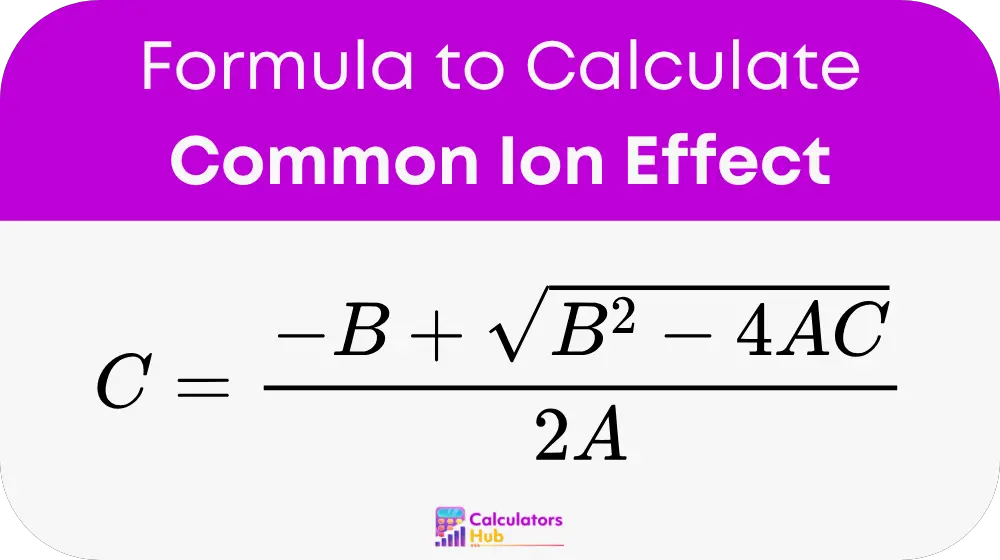
Where:
- C: Equilibrium concentration of the ion of interest (in moles per liter).
- A: Coefficient for C², which is 1 in most cases.
- B: Initial common ion concentration contributed by other dissolved compounds sharing the same ion.
- AC: -Ksp (negative value of the solubility product constant).
- Ksp: Solubility product constant of the ionic compound.
Supporting Calculations:
- Solubility Product Constant:
Ksp = [Ion_1] × [Ion_2] - Initial Common Ion Concentration:
Initial_Common_Ion_Concentration = Concentration contributed by the pre-existing common ion in the solution. - Quadratic Equation Solution:
Use the quadratic formula: C = (-B ± √(B² - 4AC)) / 2A
Only the positive root is considered since concentrations cannot be negative.
General Terms Table
The following table lists common solubility product constants (Ksp) for some ionic compounds and their corresponding equilibrium concentrations under different initial common ion concentrations. This data helps users make quick decisions without calculating each time.
| Compound | Ksp (mol²/L²) | Initial Common Ion (mol/L) | Equilibrium Concentration (mol/L) |
|---|---|---|---|
| AgCl | 1.77 × 10⁻¹⁰ | 0.01 | 1.77 × 10⁻⁸ |
| PbCl₂ | 1.7 × 10⁻⁵ | 0.05 | 1.1 × 10⁻³ |
| BaSO₄ | 1.08 × 10⁻¹⁰ | 0.02 | 5.4 × 10⁻⁶ |
| CaF₂ | 3.9 × 10⁻¹¹ | 0.01 | 1.9 × 10⁻⁵ |
| Fe(OH)₃ | 6.3 × 10⁻³⁷ | 0.001 | 7.94 × 10⁻¹² |
This table assumes the calculations are done using the quadratic formula and that no additional sources of interference exist.
Example of Common Ion Effect Calculator
Let’s calculate the equilibrium concentration for a solution of AgCl (silver chloride) with the following data:
- Ksp (AgCl): 1.77 × 10⁻¹⁰
- Initial Common Ion Concentration: 0.01 mol/L
Using the formula:
C = (-B + √(B² - 4AC)) / 2A
Here:
- A = 1
- B = 0.01
- AC = -Ksp = -1.77 × 10⁻¹⁰
Step 1: Substitute values into the quadratic formula:
C = (-0.01 + √((0.01)² - 4(1)(-1.77 × 10⁻¹⁰))) / 2(1)
Step 2: Simplify:
C = (-0.01 + √(1 × 10⁻⁴ + 7.08 × 10⁻¹⁰)) / 2
C ≈ (-0.01 + 0.01) / 2 ≈ 1.77 × 10⁻⁸ mol/L
Thus, the equilibrium concentration of Ag⁺ ions is approximately 1.77 × 10⁻⁸ mol/L.
Most Common FAQs
The common ion effect refers to the reduction in solubility of an ionic compound when a solution already contains one of its constituent ions. This effect is a result of Le Chatelier’s Principle, which states that the system will shift its equilibrium to counteract the added ion, leading to decreased solubility.
The quadratic formula is use because the solubility calculations often result in a second-degree equation. This equation accounts for the balance between the solubility product constant (Ksp), the initial ion concentration, and the equilibrium ion concentration. The formula helps find the concentration of the ion accurately.
Yes, the common ion effect can be reverse by diluting the solution or removing the common ion. This reduces the concentration of the common ion, allowing the solubility of the compound to increase.