A buffer dilution calculator is designed to help you calculate the volumes and concentrations needed to prepare buffer solutions. It ensures that experiments have consistent and repeatable conditions, which is fundamental for valid results. Using this tool can help avoid calculation errors and save time, allowing you to focus more on the experimental procedures themselves.
Formula of Buffer Dilution Calculator
At the heart of the buffer dilution calculator is the formula
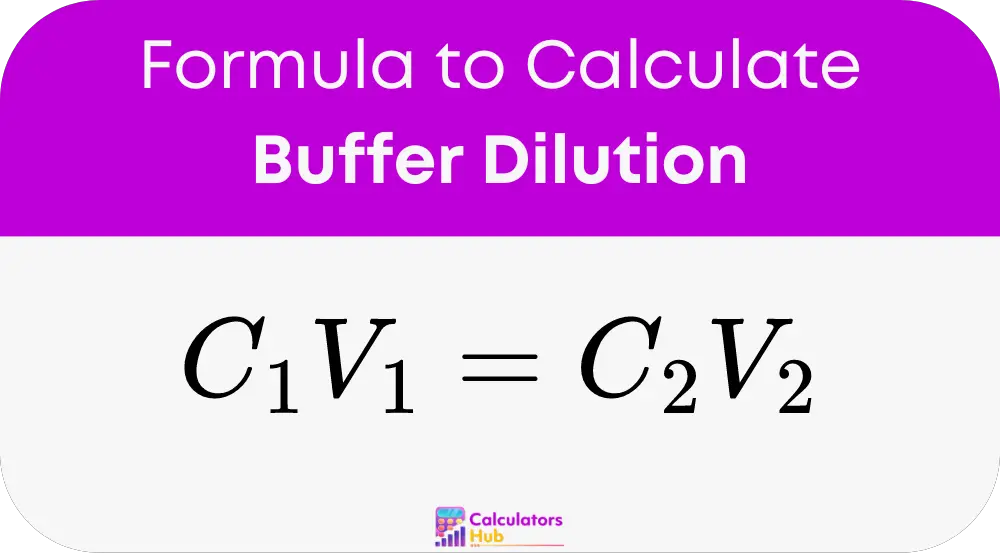
This formula helps determine any of the four variables involved in dilutions:
- C1 (initial concentration) and V1 (initial volume) represent the starting conditions of your buffer solution.
- C2 (desired final concentration) and V2 (desired final volume) represent the conditions you want to achieve.
For instance, if you know the initial concentration and volume, and you need to find out the final volume for a desired concentration, you can rearrange the formula to solve for V2: V2 = (C1 x V1) / C2 This calculation will give you the volume to which you need to dilute your initial solution to achieve the desired concentration.
Table: Buffer Dilution Quick Reference
| Buffer Solution | Initial Concentration (M) | Desired Concentration (M) | Initial Volume (mL) | Final Volume (mL) | Notes |
|---|---|---|---|---|---|
| Phosphate Buffer | 1.0 | 0.1 | 100 | 1000 | Used for biological applications |
| Tris Buffer | 0.5 | 0.1 | 500 | 2500 | Common in DNA, RNA extraction |
| Acetate Buffer | 1.0 | 0.5 | 100 | 200 | Suitable for biochemical assays |
| Citrate Buffer | 2.0 | 0.5 | 100 | 400 | Used in antigen retrieval |
Example of Buffer Dilution Calculator
Imagine you have 1 liter (1000 mL) of a buffer solution at 1 M concentration, and you need to dilute it to a 0.5 M concentration. Using our formula: C1 = 1 M, V1 = 1000 mL, C2 = 0.5 M V2 = (1 M x 1000 mL) / 0.5 M = 2000 mL This means you need to add enough solvent to reach a final volume of 2000 mL, effectively diluting your buffer to the desired concentration.
Most Common FAQs
The most common mistake is not using consistent units across all variables. Always ensure that your concentrations and volumes are in the same units before performing calculations.
Yes, the calculator is versatile for different scales, but be mindful of practical limitations in measurement accuracy, especially at very small volumes.
Several mobile apps are available that can perform buffer dilution calculations, offering convenience and portability for fieldwork or quick lab calculations.