The Bond Polarity Calculator helps you determine the polarity of a chemical bond between two atoms. Bond polarity is an important concept in chemistry because it influences the physical properties and chemical behavior of molecules. By using the electronegativity values of the atoms involved, this calculator can quickly and accurately classify the bond as nonpolar covalent, mostly nonpolar covalent, polar covalent, or ionic.
Formula of Bond Polarity Calculator
The polarity of a bond between two atoms can be determined by the difference in their electronegativities. The formula to calculate the bond polarity is as follows:
Find the Electronegativity Values: Obtain the electronegativity values (EN) of the two atoms involved in the bond from a standard electronegativity table.
Calculate the Difference: Subtract the smaller electronegativity value from the larger one to get the electronegativity difference (ΔEN):
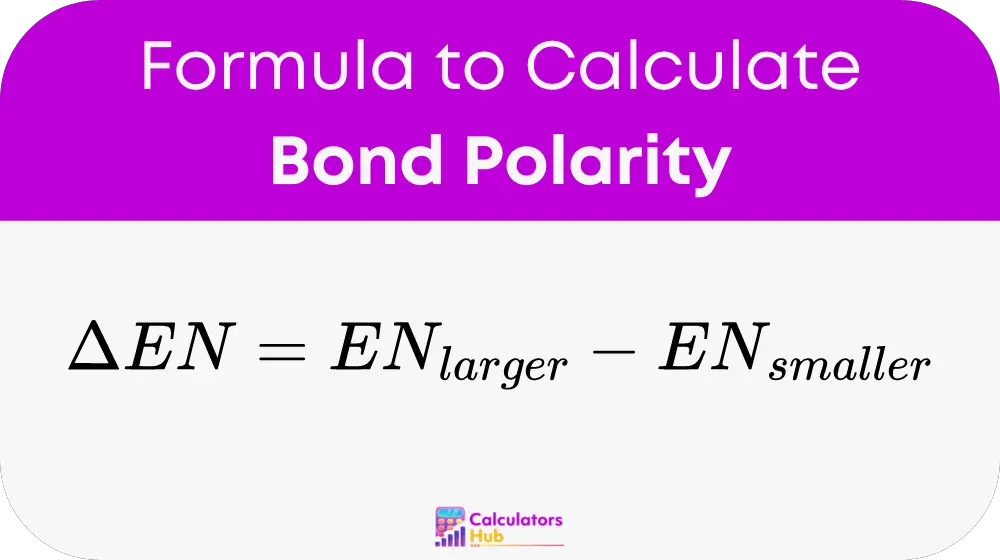
Determine the Bond Type: Use the electronegativity difference to determine the type of bond:
- If ΔEN = 0, the bond is nonpolar covalent.
- If 0 < ΔEN ≤ 0.4, the bond is mostly nonpolar covalent.
- If 0.4 < ΔEN ≤ 1.7, the bond is polar covalent.
- If ΔEN > 1.7, the bond is ionic.
General Terms Table
| Electronegativity Difference (ΔEN) | Bond Type |
|---|---|
| 0 | Nonpolar Covalent |
| 0 < ΔEN ≤ 0.4 | Mostly Nonpolar Covalent |
| 0.4 < ΔEN ≤ 1.7 | Polar Covalent |
| 1.7 | Ionic |
Example of Bond Polarity Calculator
Let’s take an example to understand how the Bond Polarity Calculator works.
Suppose we want to determine the bond polarity between hydrogen (H) and chlorine (Cl).
Step 1: Find the Electronegativity Values The electronegativity value of hydrogen (H) is 2.1. The electronegativity value of chlorine (Cl) is 3.0.
Step 2: Calculate the Difference ΔEN = EN (Cl) – EN (H) ΔEN = 3.0 – 2.1 ΔEN = 0.9
Step 3: Determine the Bond Type Since 0.4 < ΔEN ≤ 1.7, the bond between hydrogen and chlorine is a polar covalent bond.
Most Common FAQs
A nonpolar covalent bond is a type of chemical bond where two atoms share a pair of electrons with each other equally. This occurs when the electronegativity difference (ΔEN) between the atoms is zero.
A polar covalent bond is a type of chemical bond where a pair of electrons is unequally shared between two atoms. This occurs when the electronegativity difference (ΔEN) between the atoms is between 0.4 and 1.7.
An ionic bond is a type of chemical bond where one atom donates an electron to another atom. This occurs when the electronegativity difference (ΔEN) between the atoms is greater than 1.7.