The Bond Length Calculator helps determine the distance between two bonded atoms in a molecule. Bond length is a crucial concept in chemistry because it directly influences the physical and chemical properties of molecules. A shorter bond length often indicates a stronger bond, while a longer bond suggests a weaker one. This tool simplifies the process of estimating bond lengths by using the covalent radii of the atoms involved in the bond.
The bond length is particularly useful for chemists and researchers studying molecular structure, bonding interactions, and molecular dynamics. It’s also valuable for students learning about molecular geometry and covalent bonding.
Formula of Bond Length Calculator
The bond length between two atoms in a covalent bond can be approximated using the following formula:
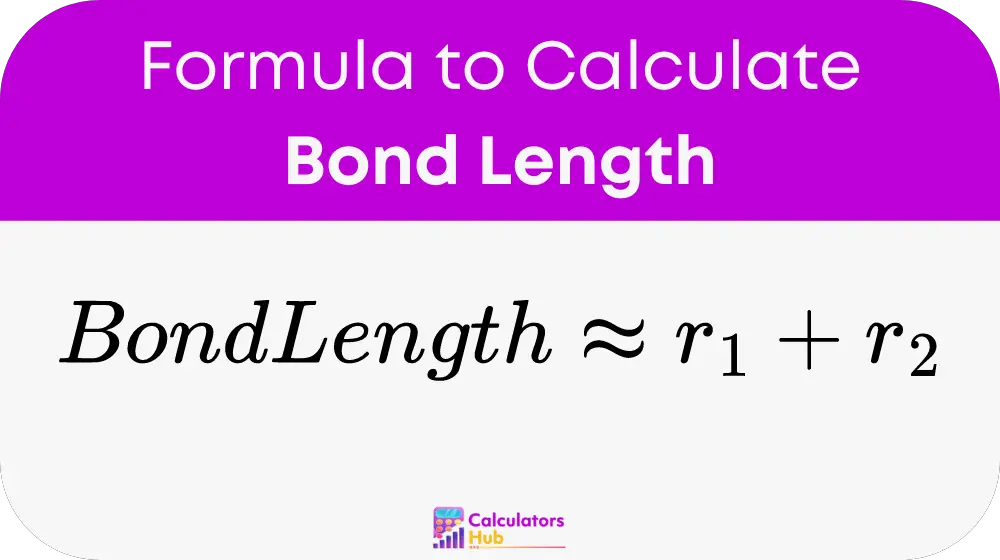
Where:
- r₁ is the covalent radius of Atom 1 (measured in picometers or angstroms).
- r₂ is the covalent radius of Atom 2 (measured in picometers or angstroms).
Key Terms:
- Bond Length: The distance between the nuclei of two bonded atoms in a molecule.
- Covalent Radius: The approximate radius of an atom’s outermost electron cloud when involved in a covalent bond.
- Picometer (pm): A unit of length commonly used to measure atomic-scale distances, where 1 picometer equals 10⁻¹² meters.
- Angstrom (Å): Another unit used to measure atomic distances, where 1 angstrom equals 10⁻¹⁰ meters.
The bond length depends on the size of the atoms involved and their covalent radii. The larger the atoms, the longer the bond.
General Reference Table for Bond Lengths
Here’s a reference table of common covalent radii for atoms and their corresponding bond lengths:
| Atom | Covalent Radius (pm) | Example Molecule | Estimated Bond Length (pm) |
|---|---|---|---|
| Hydrogen (H) | 37 | H₂ | 74 |
| Carbon (C) | 77 | C-C (ethane) | 154 |
| Oxygen (O) | 66 | O-O (oxygen gas) | 132 |
| Nitrogen (N) | 71 | N-N (nitrogen gas) | 142 |
| Chlorine (Cl) | 102 | Cl-Cl (chlorine gas) | 204 |
| Fluorine (F) | 64 | F-F (fluorine gas) | 128 |
This table provides useful estimates for some common bonds based on the covalent radii of the atoms involved.
Example of Bond Length Calculator
Let’s walk through an example to demonstrate how to use the Bond Length Calculator.
Scenario:
You are interested in calculating the bond length for a hydrogen molecule (H₂). Each hydrogen atom has a covalent radius of approximately 37 pm. What is the estimated bond length of the H-H bond?
- Step 1: Use the bond length formula: Bond Length ≈ r₁ + r₂
- Step 2: Plug in the covalent radii values for hydrogen: Bond Length ≈ 37 pm + 37 pm = 74 pm
So, the bond length for the hydrogen molecule (H₂) is approximately 74 picometers.
Scenario 2:
Now, let’s calculate the bond length for a chlorine molecule (Cl₂), where the covalent radius of each chlorine atom is 102 pm.
- Step 1: Use the same formula: Bond Length ≈ r₁ + r₂
- Step 2: Plug in the values for chlorine: Bond Length ≈ 102 pm + 102 pm = 204 pm
So, the bond length for the chlorine molecule (Cl₂) is approximately 204 picometers.
Most Common FAQs
Bond length is influenced by several factors, including:
The size of the atoms: Larger atoms generally have longer bond lengths.
The bond order: Single bonds are longer than double or triple bonds.
Electronegativity differences: When two atoms have different electronegativities, their bond length may be shorter due to stronger attractions.
In general, shorter bonds are stronger because the atoms are closer together, resulting in a stronger attraction between their nuclei. Conversely, longer bonds are weaker due to the greater distance between the bonded atoms, which reduces their attractive forces.
Yes, bond length can be measured using experimental techniques like X-ray crystallography or spectroscopy. These methods provide highly accurate measurements of the distance between atoms in a molecule.