This calculator simplifies the process of applying Avogadro’s Law by providing a user-friendly interface where you can input three variables to find the fourth. Whether you’re calculating initial or final volumes or mole numbers, this tool ensures accuracy and saves time, making it invaluable in both educational settings and professional labs.
Formula of Avogadro’s Law Calculator
Avogadro’s Law states that the volume of a gas is directly proportional to the number of moles present when the temperature and pressure remain constant. The relationship can be expressed mathematically as:
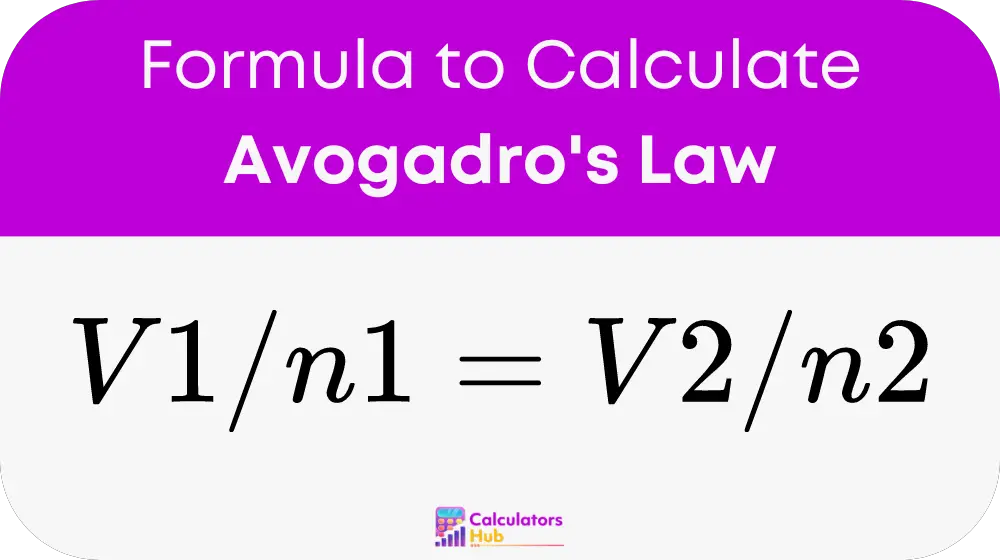
Where:
- V1 and V2 are the initial and final volumes of the gas.
- n1 and n2 are the initial and final number of moles of the gas.
This relationship implies that if you know three out of the four variables (V1, V2, n1, n2), you can easily calculate the fourth.
Practical Application and Conversion Table
Avogadro’s Law is not just a theoretical concept but has practical applications in laboratory and industrial settings. Below is a table providing general terms and conversions that are commonly used:
| Term | Description |
|---|---|
| Standard Volume | Volume of one mole of gas at standard conditions |
| Molar Volume | Volume occupied by any gas at a given condition |
These conversions are useful for quick reference without needing detailed calculations every time.
Example of Avogadro’s Law Calculator
Let’s consider a practical example: If 1 mole of a gas occupies 22.4 liters at standard temperature and pressure (STP), and we increase the amount of gas to 2 moles while keeping temperature and pressure constant, the new volume would be:
V2 = n2 * (V1 / n1) = 2 moles * (22.4 liters / 1 mole) = 44.8 liters
Most Common FAQs
Avogadro’s number, approximately 6.022×10236.022×1023 entities per mole, is use to convert between moles and particles, such as atoms or molecules.
Avogadro’s Law assumes constant temperature and pressure. Any variation in these conditions requires adjustments using combined gas laws.
Yes, it is equip to convert between common volumetric and molar units, facilitating its use in diverse scientific contexts.