The Average Abundance Calculator is an essential tool used in chemistry and physics, particularly in the study of isotopes within chemical elements. This calculator helps scientists and students calculate the average atomic mass of an element based on the abundance and mass of its isotopes. Understanding the average abundance of isotopes is crucial for fields like radiometric dating, nuclear medicine, and the study of elemental formation in the universe.
Formula of Average Abundance Calculator
To calculate the average abundance of isotopes, the following formula is used:
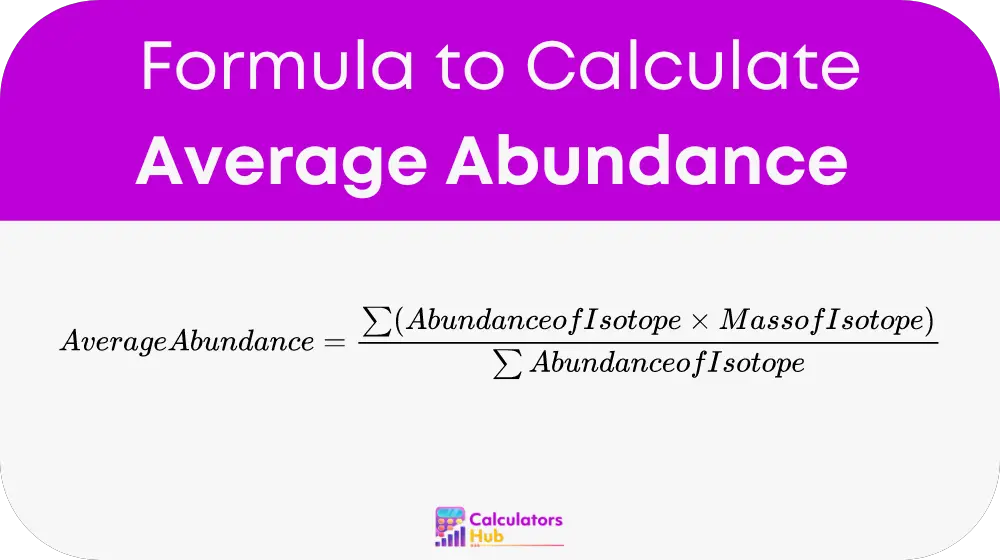
Where:
- Abundance of Isotope: The relative amount of each isotope present in a sample, often expressed as a percentage or a fraction.
- Mass of Isotope: The atomic mass of each isotope.
This formula provides a weighted average that reflects the relative proportions of each isotope within the sample.
Table of General Terms
Below is a table of key terms related to the Average Abundance Calculator:
| Term | Definition |
|---|---|
| Average Abundance | The calculated mean mass of an element based on the distribution of its isotopes and their respective masses. |
| Isotope | Variants of a particular chemical element which differ in neutron number, and consequently in nucleon number. |
| Abundance of Isotope | The proportion of a particular isotope among all isotopes of that element in a given sample. |
| Mass of Isotope | The mass of an individual isotope, typically measured in atomic mass units (amu). |
Example of Average Abundance Calculator
For example, consider an element X with two isotopes:
- Isotope X-1 has a mass of 10 amu and an abundance of 75%.
- Isotope X-2 has a mass of 11 amu and an abundance of 25%.
Using the formula, the average abundance can be calculated as follows:
Average Abundance = ((0.75 * 10) + (0.25 * 11)) / (0.75 + 0.25) = (7.5 + 2.75) / 1 = 10.25 amu
This result indicates that the average mass of element X, considering the distribution of its isotopes, is 10.25 amu.
Most Common FAQs
It is a tool used to compute the average atomic mass of an element based on the relative abundances and masses of its isotopes.
Calculating the average isotope abundance helps in various scientific fields, including chemistry and geology, to determine the composition of elements and their applications in practical scenarios.
Yes, the average abundance can vary depending on the source of the element and environmental factors, which can lead to variations in isotopic composition