The AMU to Grams Calculator is a crucial tool for scientists, particularly chemists and physicists, enabling them to convert the mass of substances from atomic mass units (AMU) to grams. This conversion is essential in the laboratory for preparing chemical reactions and understanding the macroscopic properties of materials based on their microscopic masses.
Formula of Amu To Grams Calculator
The formula to convert AMU to grams incorporates Avogadro's number, which is central to the concept of a mole in chemistry:
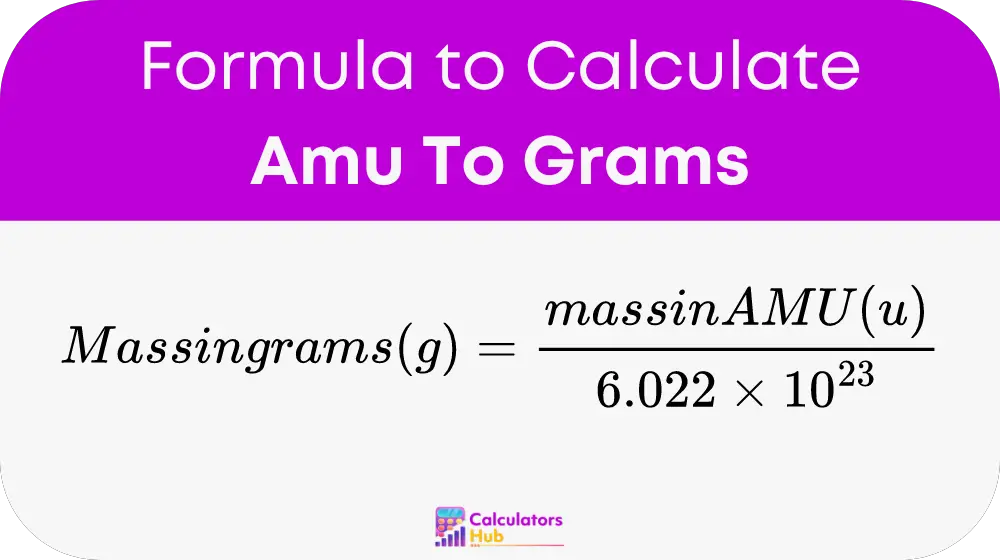
Where:
- Mass in grams (g) is the mass you are calculating.
- Mass in AMU (u) is the given mass in atomic mass units.
- 6.022 x 10^23 is Avogadro's number, representing the number of atoms or molecules in one mole.
This formula is derived from the definition of AMU and the mole concept, providing a bridge between the microscopic world of atoms and the macroscopic world of grams.
Table of Common Conversions
To facilitate easier conversions without calculations, below is a table that lists common elements and their respective weights in AMU and converted grams for a single atom:
| Element | Atomic Weight (AMU) | Weight of One Atom (grams) |
|---|---|---|
| Hydrogen (H) | 1 | 1.66 x 10^-24 |
| Carbon (C) | 12 | 1.99 x 10^-23 |
| Oxygen (O) | 16 | 2.66 x 10^-23 |
| Gold (Au) | 197 | 3.27 x 10^-22 |
This table provides a quick reference to understand the mass of individual atoms in grams, which is particularly useful in quantitative chemical analysis.
Example of Amu To Grams Calculator
For an illustrative example, consider calculating the mass in grams of one mole of carbon atoms. Carbon has an atomic mass of approximately 12 AMU. Using the formula:
Mass in grams = 12 AMU / (6.022 x 10^23)
This calculation shows that one mole of carbon atoms weighs 12 grams. Reflecting the direct relationship between atomic mass and molar mass.
Most Common FAQs
AMU, or atomic mass unit, is a standard unit of mass that quantifies the mass of atoms or molecules on a scale where one AMU is defined as one-twelfth the mass of a carbon-12 atom. It is essential for atomic-level calculations and understanding the chemical properties of elements.
Avogadro's number (6.022 x 10^23) is the number of units in one mole of any substance. Which bridges the microscopic measurements in AMU to macroscopic measurements in grams, facilitating practical chemical calculations.
Yes, this calculator can also be used for molecules by summing the atomic masses of all the atoms in the molecule to get the total molecular mass in AMU. Which can then be convert to grams using the same formula.