The Resting Membrane Potential Calculator is a specialized tool designed to calculate the equilibrium potential (also known as the Nernst potential) for a particular ion based on its concentration inside and outside the cell. This calculation is vital for understanding cellular functions in both normal and pathological states. The equilibrium potential is a critical component in determining the overall membrane potential of a cell, which influences nerve impulse transmission and muscle contraction.
Formula of Resting Membrane Potential Calculator
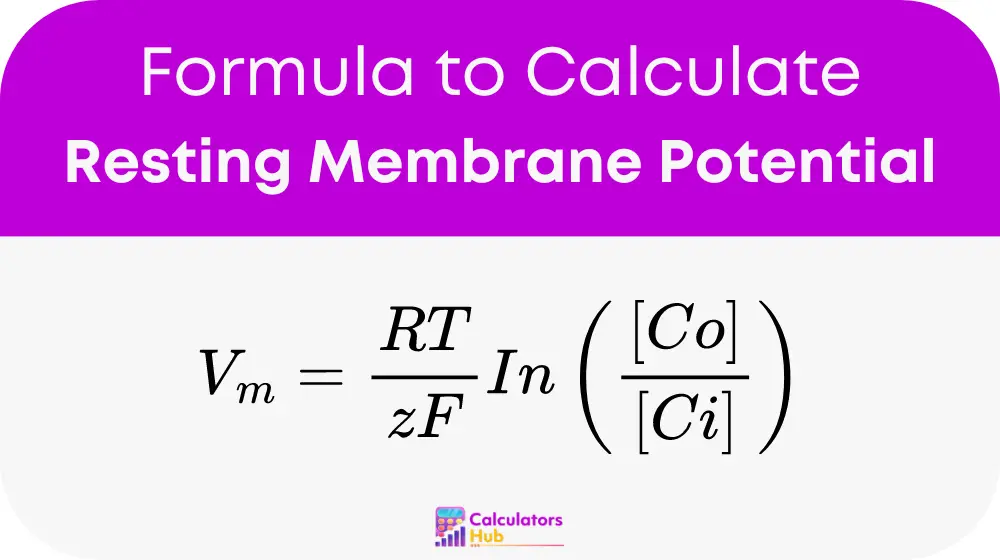
Where:
Vm= equilibrium potential for the ion (in volts, V)R= universal gas constant (8.314 joules per Kelvin per mole, J/K/mol)T= absolute temperature (in Kelvin, K = °C + 273.15)z= valence of the ion (e.g., 1 for potassium (K+), 2 for calcium (Ca2+))F= Faraday’s constant (96485 coulombs per mole, C/mol)ln= natural logarithm (base-e logarithm)[Co]= concentration of the ion outside the cell (in moles per liter, mol/L)[Ci]= concentration of the ion inside the cell (in moles per liter, mol/L)
This formula allows the calculator to determine the potential difference caused by a particular ion under physiological conditions.
Example of Resting Membrane Potential Calculator
To illustrate how to use the Resting Membrane Potential Calculator, consider a scenario where you need to find the equilibrium potential for potassium (K+) at physiological temperature (37°C), with external potassium concentration of 5 mM and internal concentration of 140 mM.
Inputs:
- Ion: Potassium (K+)
- Temperature: 37°C (which is 310.15 K)
- External concentration ([Co]): 5 mM
- Internal concentration ([Ci]): 140 mM
Calculation:
Vm = (8.314 * 310.15 / (1 * 96485)) * ln(5 / 140)
= 0.0267 * ln(0.0357)
= 0.0267 * (-3.333) = -0.089 V
Therefore, the equilibrium potential for potassium under these conditions is approximately -89 mV, indicating a typical value for this ion in many cells.
Conversion Table for Common Ions
| Ion | External Concentration ([Co]) | Internal Concentration ([Ci]) | Equilibrium Potential (Vm) |
|---|---|---|---|
| Potassium (K+) | 5 mM | 140 mM | -89 mV |
| Sodium (Na+) | 145 mM | 12 mM | +66 mV |
| Calcium (Ca2+) | 2.5 mM | 0.0001 mM | +123 mV |
| Chloride (Cl-) | 110 mM | 4 mM | -86 mV |
This table provides a quick reference for the most commonly encountered ions in physiological studies.
Most Common FAQs
The resting membrane potential is crucial for the transmission of nerve impulses and muscle contractions. It establishes a state of readiness whereby a cell can activate in response to stimuli, essential for the functioning of nerves and muscles.
The calculator is highly accurate provided that the correct and precise values for ion concentration and temperature are inputted. It uses the Nernst equation, a fundamental principle in electrochemistry and physiology, ensuring reliability.
Yes, by adjusting the ion concentrations or temperature in the calculator, one can predict the changes in membrane potential in response to various physiological or experimental conditions.